Preparation method of spinel-type LiMn2O4 cathode material coated by cobalt-aluminum composite metal oxide
A spinel type, composite metal technology, applied in battery electrodes, structural parts, electrical components, etc., can solve problems such as poor coating effect, and achieve the effect of good electrochemical cycle stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
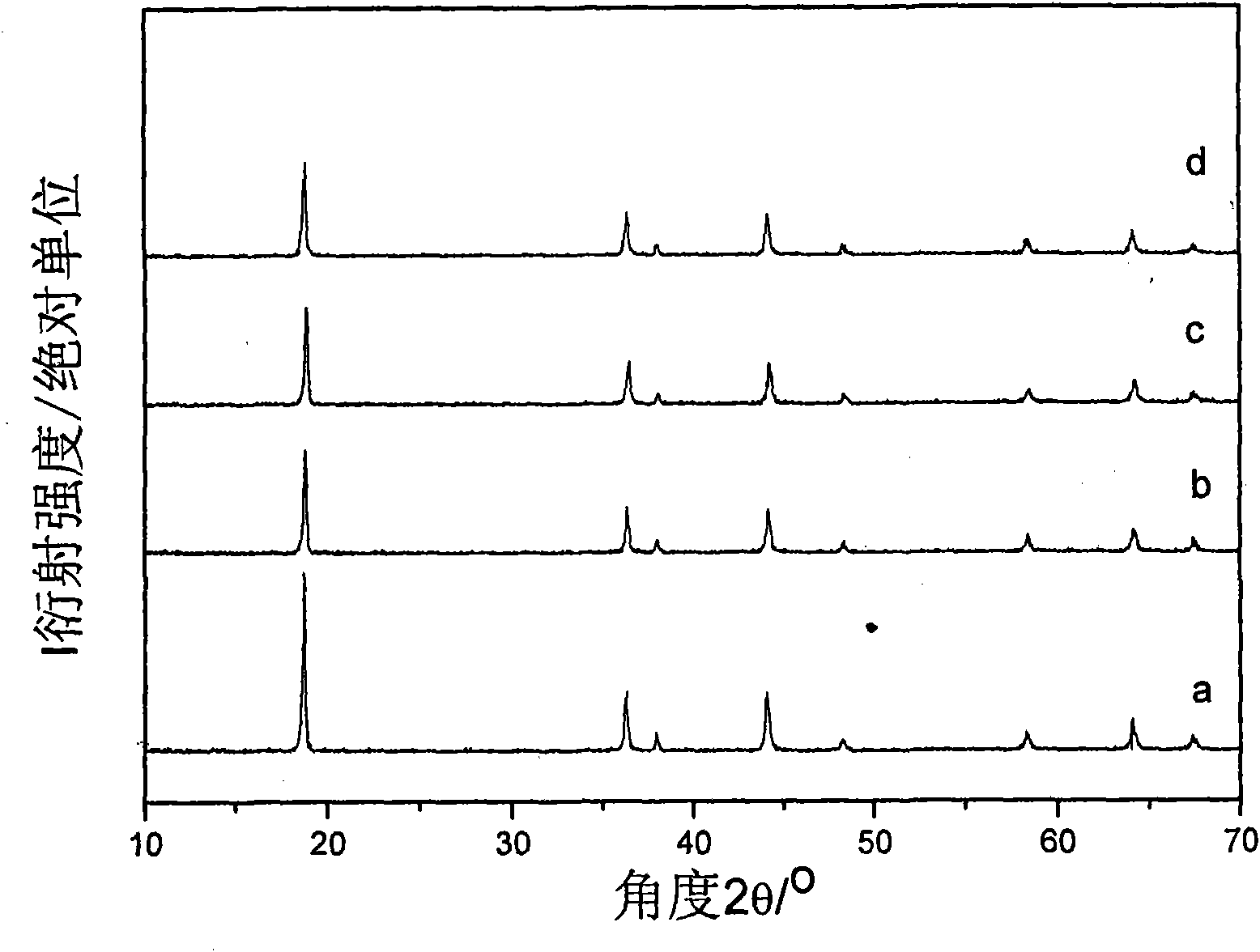
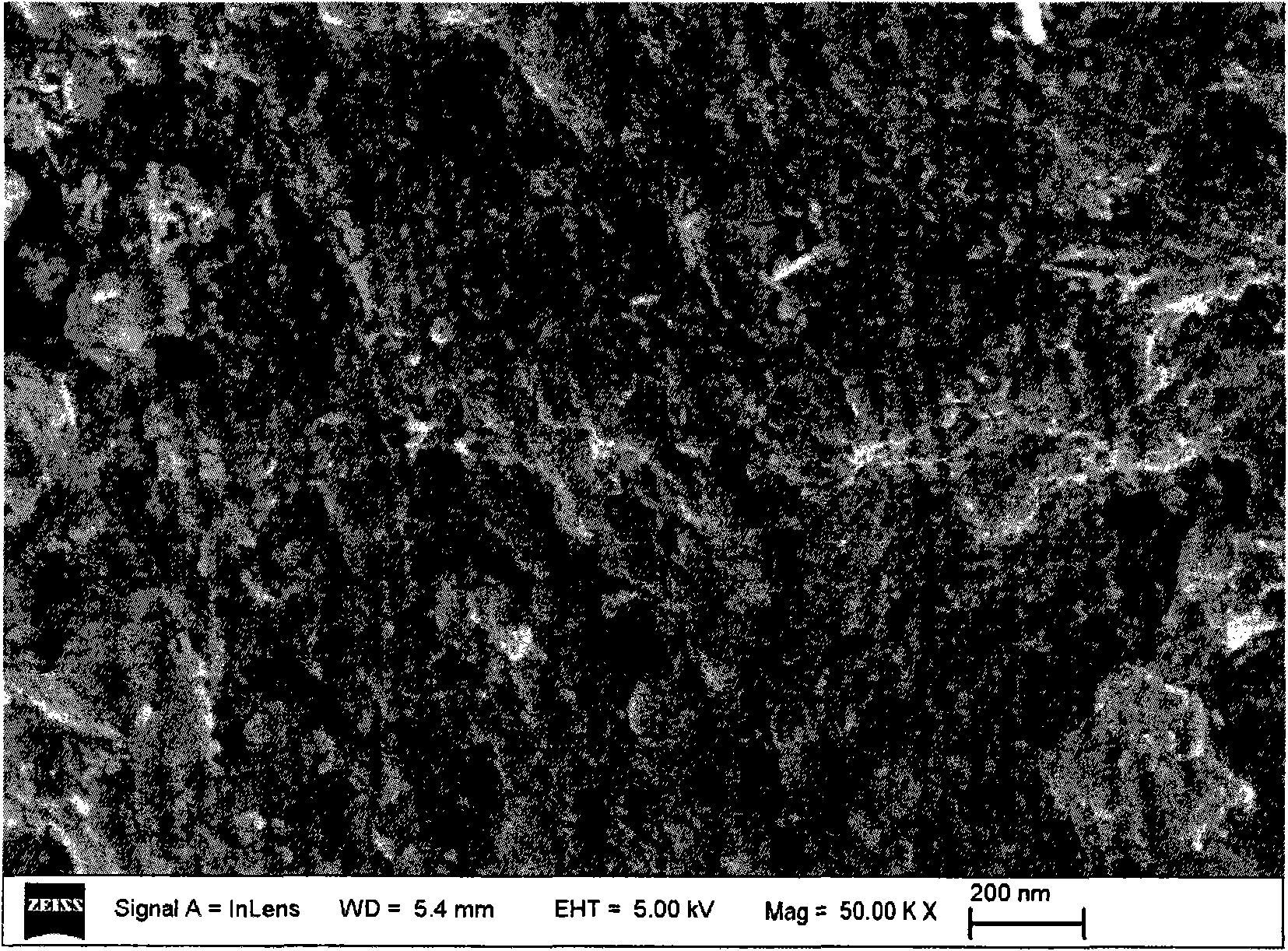
Image
Examples
Embodiment 1
[0025] Weigh 4.44g Co(NO 3 ) 2 ·6H 2 O and 2.07g Al(NO 3 ) 3 9H 2 O was dissolved in 100mL deionized water to obtain 0.15mol / L Co(NO 3 ) 2 and 0.055mol / L Al(NO 3 ) 3 mixed salt solution; weigh 1.87g LiOH·H 2 O was dissolved in 100mL deionized water to obtain a 0.45mol / L LiOH solution; 30g LiMn 2 o 4 Add it into 100mL deionized water, stir mechanically to obtain a suspension; add 50mL Co(NO 3 ) 2 and Al(NO 3 ) 3 mixed salt solution, and LiOH solution was added dropwise to keep the pH of the suspension at 10.5; after crystallization for 1 hour, filter to obtain cobalt-aluminum hydrotalcite-coated LiMn 2 o 4 Precursor. The remaining 50mL Co(NO 3 ) 2 and Al(NO 3 ) 3 The mixed salt solution and the remaining LiOH solution were added to the colloid mill with a rotation speed of 3500 rpm and reacted for 3 minutes to obtain a cobalt-aluminum hydrotalcite suspension; the cobalt-aluminum hydrotalcite prepared above was coated with LiMn 2 o 4 The precursor was added...
Embodiment 2
[0029] Weigh 11.88g Co(NO 3 ) 2 ·6H 2 O and 7.65gAl(NO 3 ) 3 9H 2 O was dissolved in 60mL deionized water to obtain 0.68mol / L Co(NO 3 ) 2 and 0.34mol / L Al(NO 3 ) 3 mixed salt solution; weigh 5.45g LiOH·H 2 O was dissolved in 100mL deionized water to obtain a 1.3mol / L LiOH solution; 200g LiMn 2 o 4 Add it into 100mL deionized water, stir mechanically to obtain a suspension; add 30mL Co(NO 3 ) 2 and Al(NO 3 ) 3 mixed salt solution, and LiOH solution was added dropwise to keep the pH value of the suspension at 9.0; after crystallization for 0.5 hours, filter to obtain cobalt-aluminum hydrotalcite-coated LiMn 2 o 4 Precursor. The remaining 30mL Co(NO 3 ) 2 and Al(NO 3 ) 3 The mixed salt solution and the remaining LiOH solution were added to the colloid mill with a rotation speed of 3000 rpm and reacted for 5 minutes to obtain a cobalt-aluminum hydrotalcite suspension; the cobalt-aluminum hydrotalcite prepared above was coated with LiMn 2 o 4 The precursor was...
Embodiment 3
[0032] Weigh 5.93g Co(NO 3 ) 2 ·6H 2 O and 2.75g Al(NO 3 ) 3 9H 2 O was dissolved in 50mL deionized water to obtain 0.41mol / L Co(NO 3 ) 2 and 0.15mol / L Al(NO 3 ) 3 mixed salt solution; weigh 2.50g LiOH·H 2 O was dissolved in 50mL deionized water to obtain a 1.2mol / L LiOH solution; 60g LiMn2 o 4 Added into 50mL deionized water, mechanically stirred to obtain a suspension; dropwise added 20mL Co(NO 3 ) 2 and Al(NO 3 ) 3 mixed salt solution, and LiOH solution was added dropwise to keep the pH of the suspension at 12.0; after crystallization for 3 hours, filter to obtain cobalt-aluminum hydrotalcite-coated LiMn 2 o 4 Precursor. The remaining 30mL Co(NO 3 ) 2 and Al(NO 3 ) 3 The mixed salt solution and the remaining LiOH solution were added to the colloid mill with a rotation speed of 4000 rpm and reacted for 1 minute to obtain a cobalt-aluminum hydrotalcite suspension; the cobalt-aluminum hydrotalcite prepared above was coated with LiMn 2 o 4 The precursor is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com