Quinacridone-thiophene derivative, preparation method and application thereof
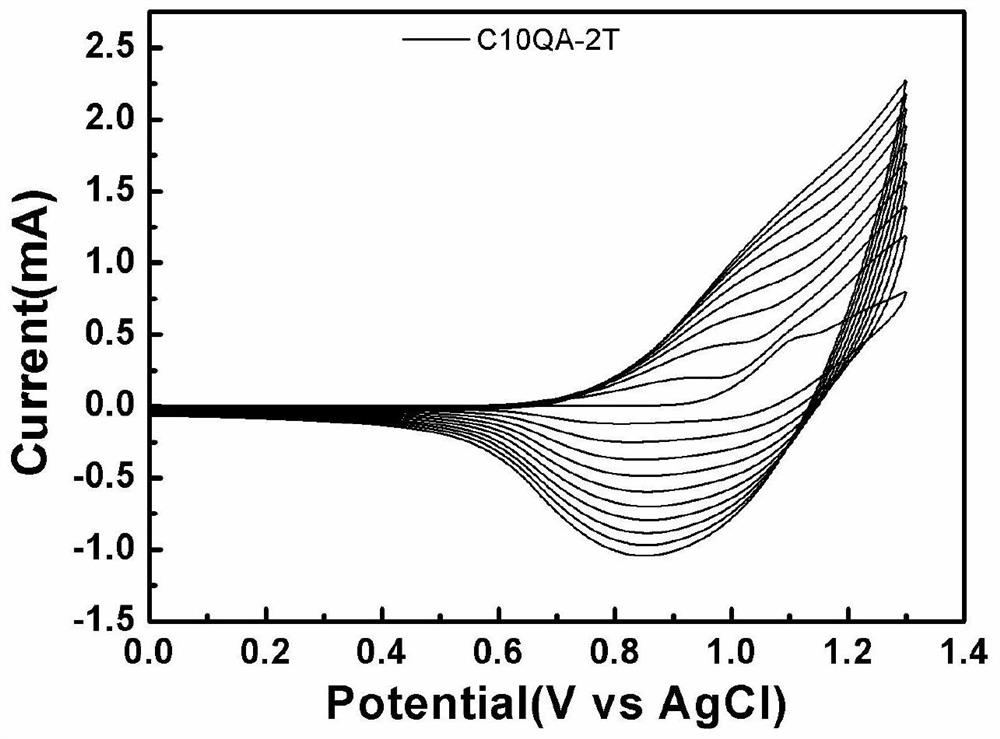
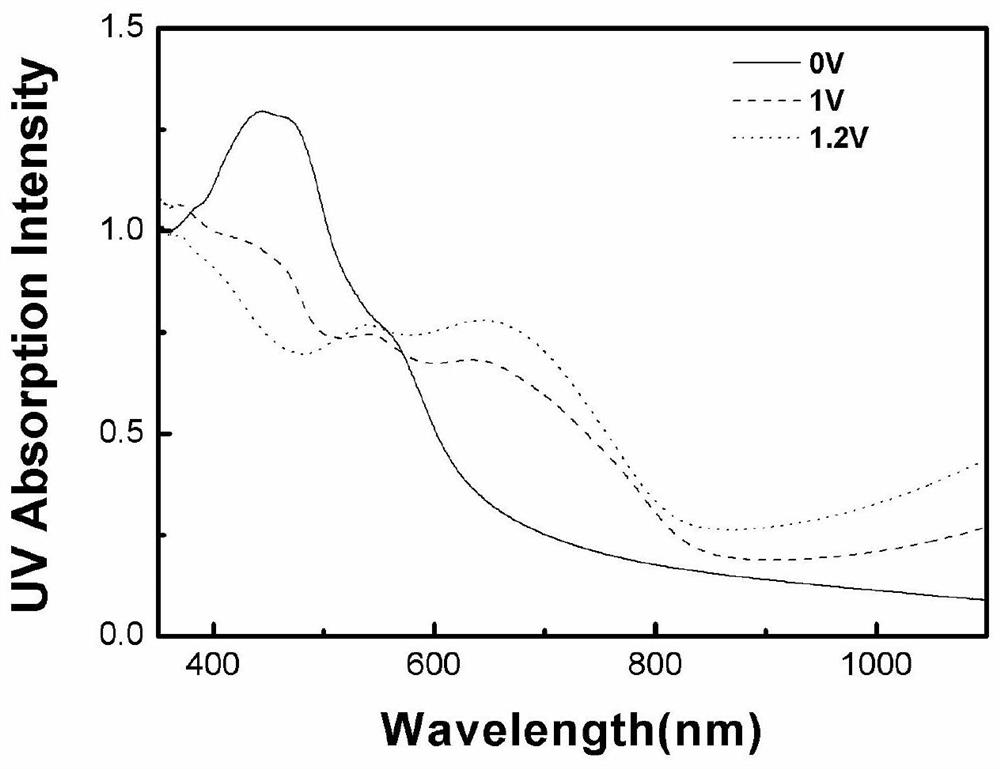
A technology of thiophene derivatives and quinacridone, which is applied in the field of quinacridone-thiophene derivatives and its preparation, can solve the problems of slow response speed, limited application range, single color display, etc., and achieve good electrochemical cycle stability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
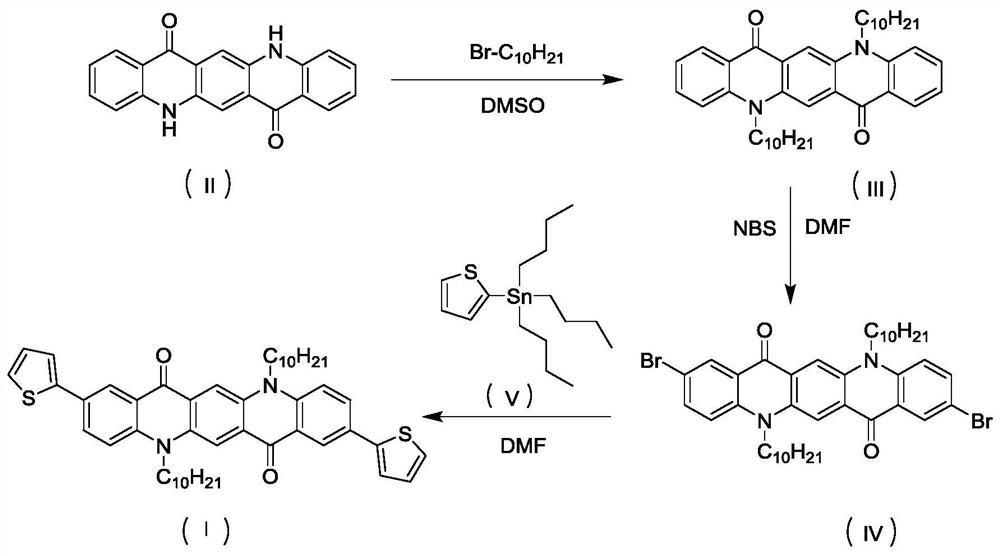
[0048] Example 1: Synthesis of C10QA
[0049]
[0050] Quinacridone 3.12g (0.01mol), 1-bromodecane 8.84g (0.04mol), sodium hydroxide 1.6g (0.04mol), tetrabutylammonium bromide 1.3g (0.004mol), dimethyl ethylene Add 30mL of sulfone to a 100mL single-necked flask in sequence, and stir at room temperature for 24 hours. After the reaction, extract with water and dichloromethane three times. After the extract is concentrated, use anhydrous sodium sulfate to remove water, and perform column chromatography purification, using silica gel as the stationary phase. Dichloromethane and petroleum ether were used as the mobile phase. The eluate containing the target compound was collected, and the solvent was removed by rotary evaporation and dried to obtain orange-red solid C10QA with a yield of 85%. The characterization structure of the confirmed substance is as follows: 1 HNMR (500MHz, CDCl 3 )δ 8.82(s, 2H), 8.61(dd, 2H), 7.80(td, 2H), 7.56(d, 2H), 7.30(t, 2H), 4.55(m, 4H), 2.0(m, 4...
Embodiment 2
[0051] Example 2: Synthesis of C10QA-2Br
[0052]Add 1.19g (0.002mol) of C10QA and 1.25g (0.007mol) of NBS into a 100mL two-necked flask, add 10mL of N,N-dimethylformamide (DMF) under nitrogen protection, heat to reflux, and react in the dark for 24 hours , after the reaction, extract three times with water and dichloromethane, concentrate the extract and remove water with anhydrous sodium sulfate, carry out column chromatography purification, use silica gel as the stationary phase, dichloromethane and petroleum ether as the mobile phase, collect the The eluate of the compound was evaporated to remove the solvent and dried to obtain orange-red solid C10QA-2Br with a yield of 80%. The characterization structure of the confirmed substance is as follows: 1 H NMR (500MHz, CDCl 3 )δ8.78(s, 2H), 8.70(s, 2H), 7.85(d, 2H), 7.45(d, 2H), 4.55(m, 4H), 2.0(m, 4H), 1.64(m, 4H ), 1.47(m, 4H), 1.42-1.20(m, 20H), 0.90(t, 6H).
Embodiment 3
[0053] Example 3: Synthesis of C10QA-2T
[0054] Add 0.32g (0.4mmol) of C10QA-2Br and a small amount of tetrakis(triphenylphosphine)palladium to a 100mL two-necked flask, and add 0.64g (0.16mmol) of 2-tributylstannylthiophene and 10mL of DMF under nitrogen protection. , heated to reflux for 12 hours. After the reaction, extract three times with water and dichloromethane, concentrate the extract, remove water with anhydrous sodium sulfate, and perform column chromatography purification, using silica gel as the stationary phase, dichloromethane and petroleum ether as the mobile phase, and collect the target compound. The eluate was evaporated to remove the solvent and dried to obtain pink solid C10QA-2T with a yield of 60%. The characterization structure of the confirmed substance is as follows: 1 H NMR (500MHz, CDCl 3 )δ 8.77(s, 2H), 8.69(s, 2H), 7.85(d, J=9.2Hz, 2H), 7.45(d, J=8.4 Hz, 2H), 4.52(s, 4H), 2.24(m , 4H), 1.60 (m, 4H), 1.52-1.20 (m, 24H), 0.90 (t, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com