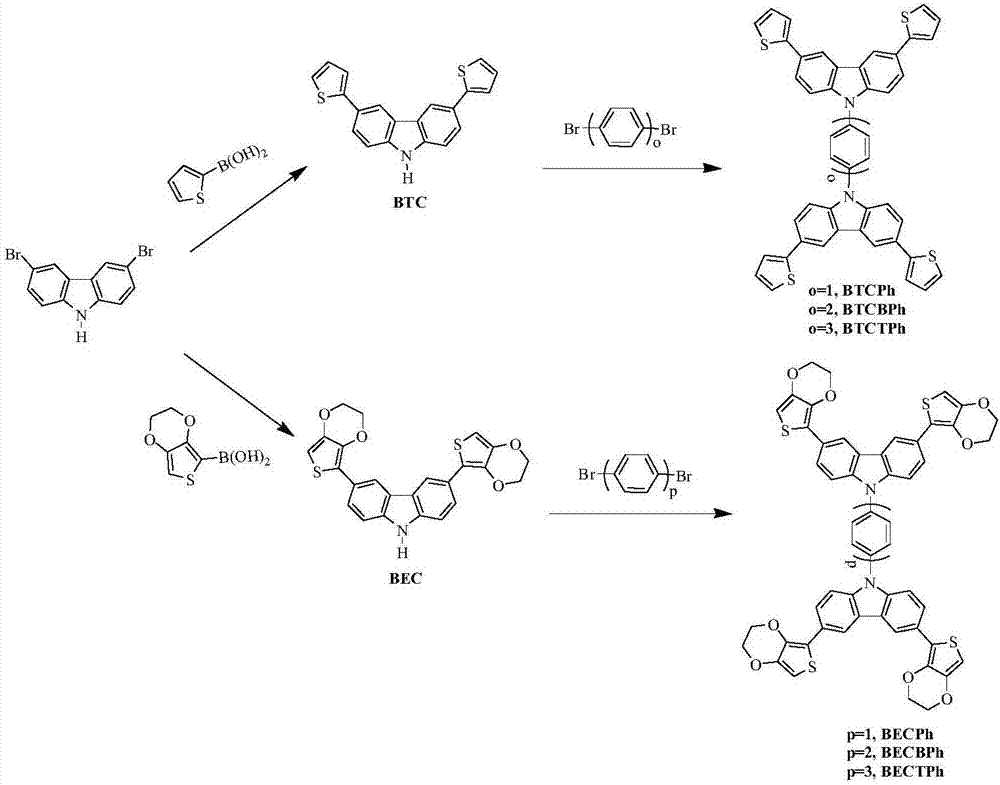
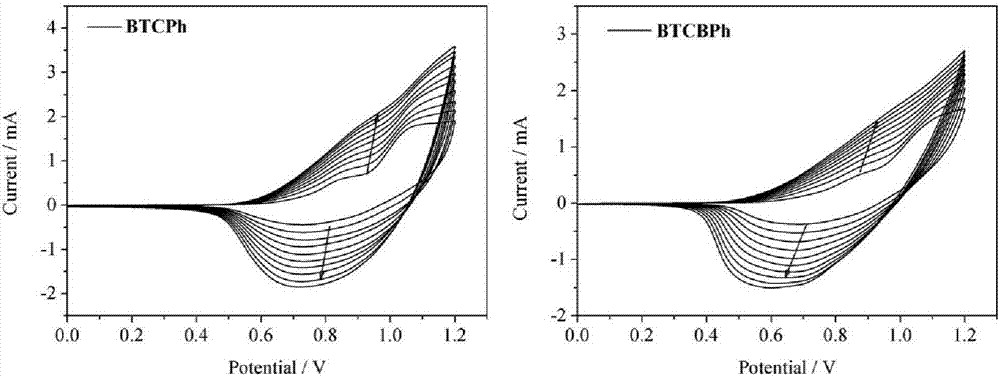
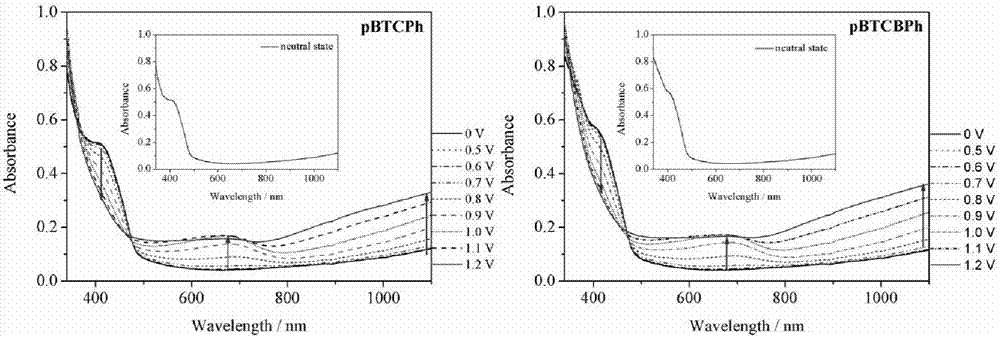
Thiophene-carbazole-thiophene derivative, preparation method and applications thereof
A thiophene derivative, thiophene technology, applied in chemical instruments and methods, instruments, color-changing fluorescent materials, etc., can solve the problems of limited large-scale application, monotonous color types, low coloring efficiency, etc., and achieve good electrochemical cycle stability, The effect of fast response time, reasonable optical contrast
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Synthesis of 4,4'-bis(3,6-dithiophene-9hydro-carbazole-9)-1,1'-biphenyl
[0041] (1) Under nitrogen protection, 3,6-dibromocarbazole (1.43g, 3mmol), 2-thiophene boronic acid (0.896g, 7mmol), tetrakis (triphenylphosphine) palladium (0.2mmol) and sodium carbonate (2.0M, 5mL) was dissolved in a mixed solvent of tetrahydrofuran (20mL) and toluene (30mL), and the system was refluxed for 48 hours. After the system was cooled, it was extracted with deionized water and dichloromethane, and the obtained organic phase was added with anhydrous MgSO 4 After drying, it was concentrated under reduced pressure, and then separated and purified by column chromatography. The stationary phase was 300-400 mesh silica gel, and the mobile phase was dichloromethane / petroleum ether (volume ratio 1:3), and finally the intermediate product 3 was obtained as a white solid. , 0.68g of 6-dithiophenecarbazole compound, the yield is 68%. 1 H NMR (500MHz, CDCl 3 )δ:8.35(s,2H),8.12(s,H),7....
Embodiment 2
[0043] Example 2 Synthesis of 4,4'-bis(3,6-dithiophene-9hydro-carbazole-9)-1,1'-benzene
[0044] The synthesis method is the same as in Example 1, except that in step (2), 1,4-dibromobiphenyl is replaced by 1,4-dibromobenzene (235.90g / mol, 2mmol, 0.4718g), and finally obtained The target product is 0.62g, and the yield is 42%.
[0045] 4,4'-bis(3,6-dithiophene-9hydro-carbazole-9)-1,1'-benzene C 46 h 28 N 2 S 4 The MS (EI) characterization found to be 736.11.
Embodiment 3
[0046] Example 3 Synthesis of 4,4'-bis(3,6-dithiophene-9hydro-carbazole-9)-1,1'-terphenyl
[0047] The synthesis method is the same as in Example 1, except that in step (2), 1,4-dibromobiphenyl is replaced by 1,4-dibromoterphenyl (388.10g / mol, 2mmol, 0.7762g), and finally 0.55 g of the target product was obtained with a yield of 31%.
[0048] The MS (EI) characterization of 4,4'-bis(3,6-dithiophene-9hydro-carbazole-9)-1,1'-terphenyl C58H36N2S4 was found to be 888.01.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com