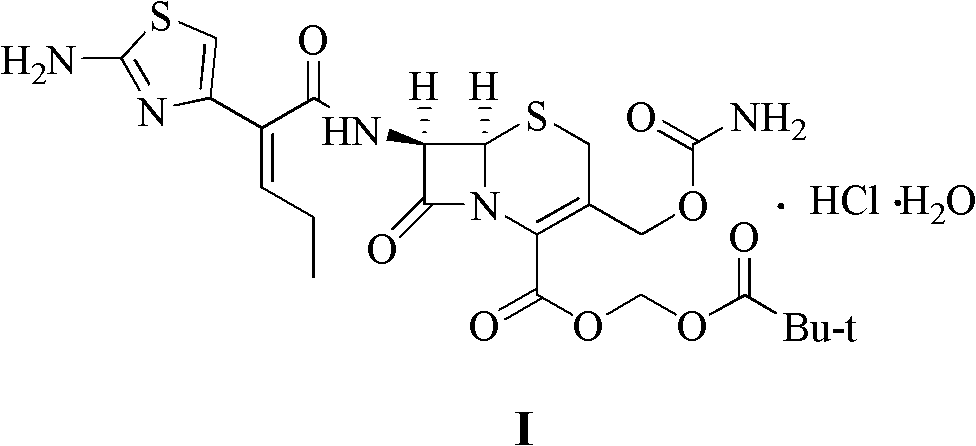
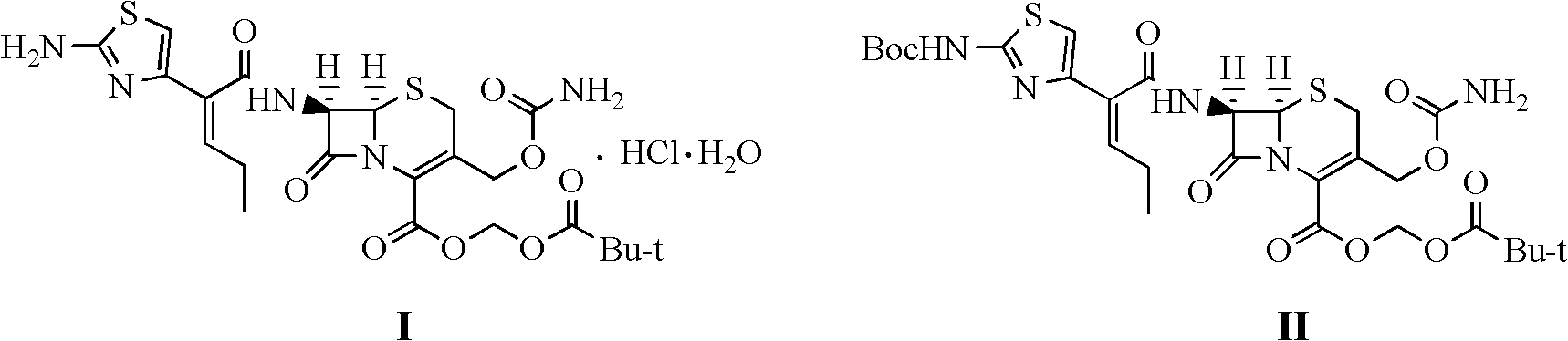Method for preparing cefcapene pivoxil hydrochloride
A technology of cefcapine and picoxil, applied in the direction of organic chemistry, can solve the problems of serious environmental pollution and high cost, and achieve the effects of high yield, low cost, great implementation value and potential social and economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 30g Boc-protected cefcarpine pivoxil was dissolved in 300g 2-methyltetrahydrofuran, and 30g 85% phosphoric acid was added dropwise at -15~-10°C, and after stirring and reacting for 4 hours, it was adjusted to pH = 7.0, separate the water layer, and concentrate the organic layer to dryness under reduced pressure to obtain an oil, add 30g of ethanol to dissolve, add 44g of 15% hydrochloric acid solution dropwise at 10-15°C to form a salt, keep stirring for 3 hours, filter, and take the filter cake for use 90 g of 50% ethanol aqueous solution was rinsed, and the washed filter cake was vacuum-dried at 45° C. to constant weight to obtain 22.0 g of cefcarpine pivoxil hydrochloride with a yield of 78.7% and a HPLC purity of 99.27%.
Embodiment 2
[0026] Dissolve 30g of Boc-protected cefcarpine pivoxil in 180g of ethyl acetate, add 45g of 80% phosphoric acid dropwise at -5~0°C, stir and react for 2.5 hours, adjust to pH=7.0 with 10% sodium hydroxide, divide Remove the water layer, and concentrate the organic layer to dryness under reduced pressure to obtain an oily substance, add 90g of ethanol to dissolve it, add 98g of 5% hydrochloric acid solution dropwise at 15-18°C to form a salt, keep stirring for 4 hours, filter, and take the filter cake with 60g of 30% hydrochloric acid Rinse with ethanol aqueous solution, and vacuum-dry the washed filter cake at 45° C. to constant weight to obtain 24.5 g of cefcarpine pivoxil hydrochloride with a yield of 87.5% and a purity of 99.25% by HPLC.
Embodiment 3
[0028] Dissolve 30g of Boc-protected cefcarpine pivoxil in 150g of 1,2-dichloroethane, add 90g of 60% phosphoric acid dropwise at 5-10°C, stir and react for 2 hours, then adjust the pH with 10% sodium hydroxide =7.0, remove the water layer, and concentrate the organic layer to dryness under reduced pressure to obtain an oily substance, add 30g of methanol to dissolve, add 32g of 5% hydrochloric acid solution dropwise at 15-20°C to form a salt, keep stirring for 4 hours, filter, and take the filter cake Rinse with 120 g of 10% ethanol aqueous solution, and vacuum-dry the washed filter cake at 45° C. to constant weight to obtain 21.7 g of cefcarpine pivoxil hydrochloride with a yield of 77.6% and an HPLC purity of 99.33%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com