Pharmaceutical composition with functions of hemangiectasis and beta 1 receptor retardation
A technique for vasodilation and blockade, applied in the field of designing medicine, can solve the problems of increasing the difficulty of preparation, producing a lot of dust, and failing to meet pharmacopoeia standards.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Nebivolol Hydrochloride 5.45g
[0021] PVPK30 5.45g
[0022] Lactose 89.1g
[0023] Croscarmellose Sodium 12.00g
[0024] Magnesium Stearate 1.00g
[0025] Silica 3.00g
[0026] 10% PVPK30 ethanol solution appropriate amount
[0027]
[0028] Make 1000 tablets
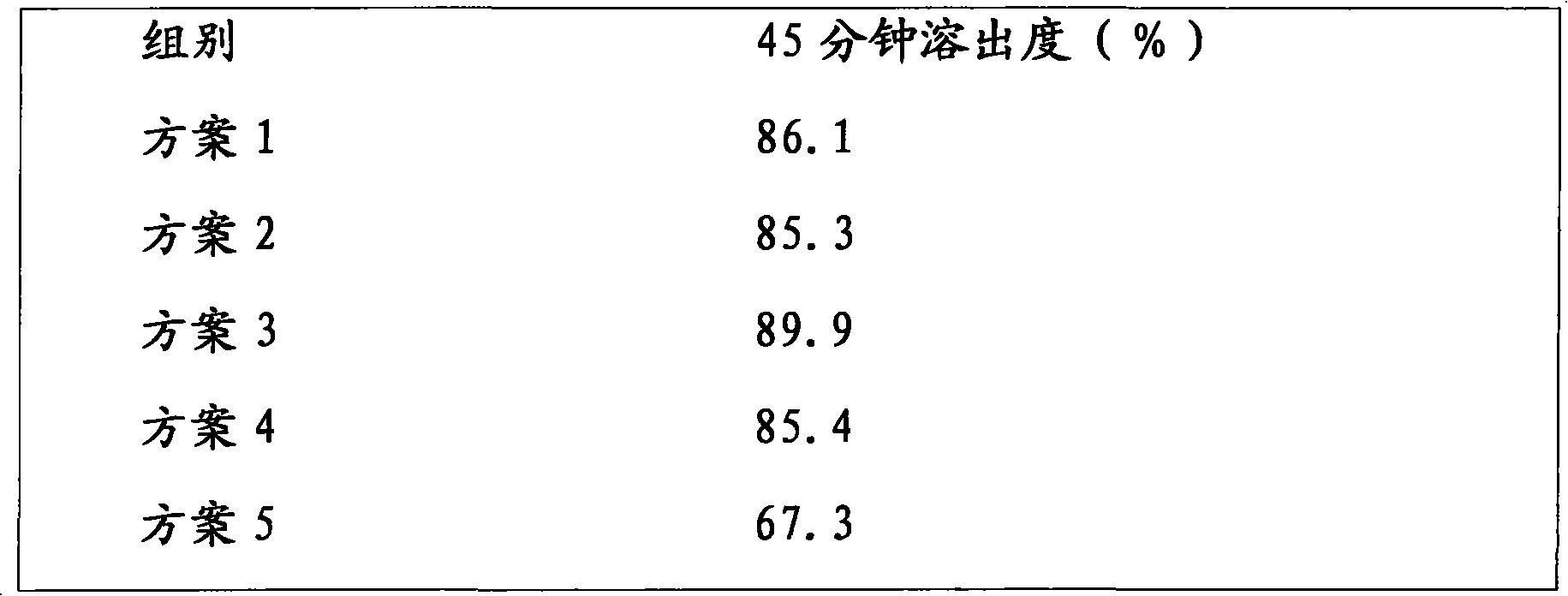
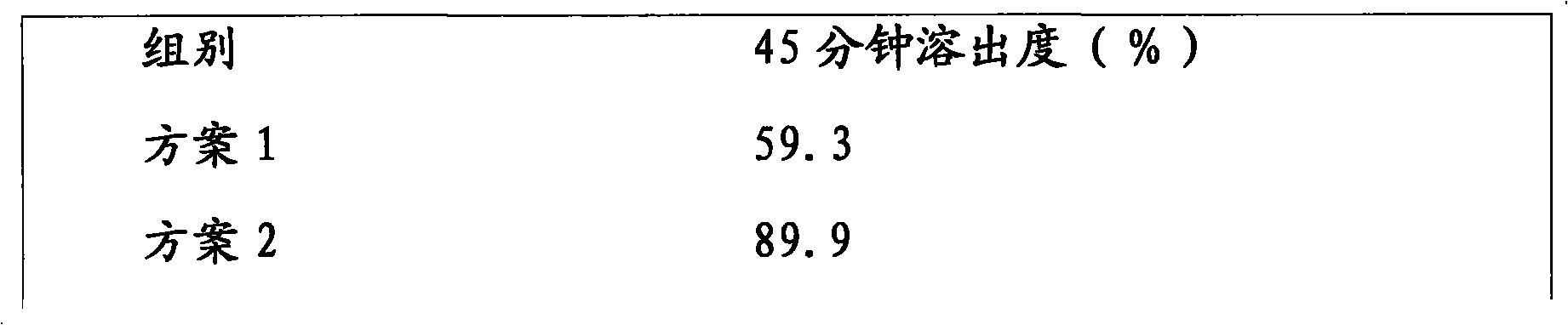
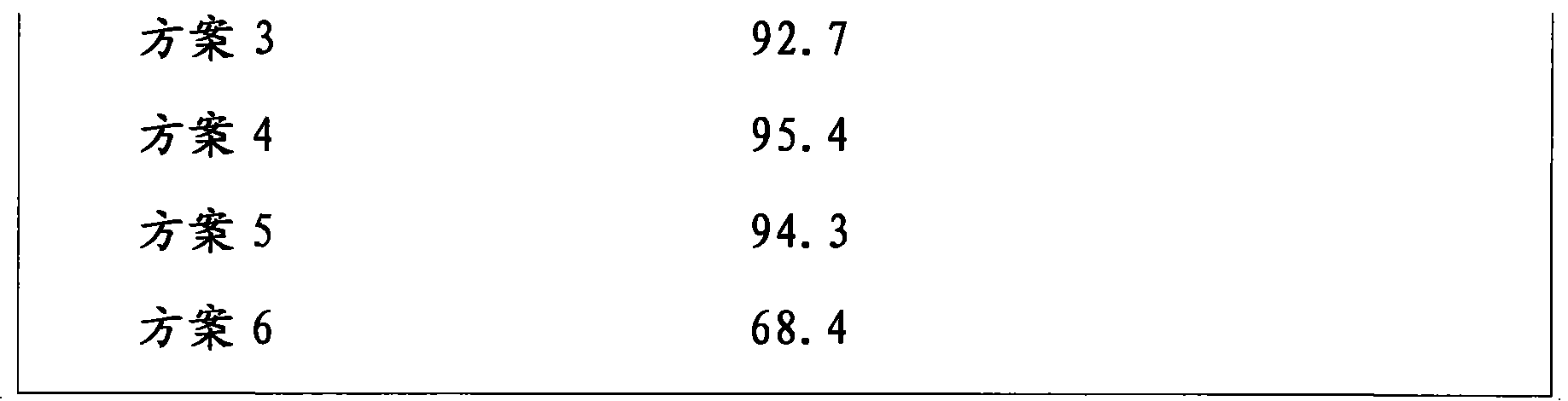
[0029] Use 10% PVPK30 ethanol solution to make lactose into soft material, pass through a 40-mesh sieve to granulate, dry at 50-60°C, and granulate with a 40-mesh sieve; put nebivolol hydrochloride and PVPK30 in a planetary ball mill, mix and grind at 500rpm for 15min. After the above materials are mixed evenly, 12% croscarmellose sodium, 1% magnesium stearate and 2.5% silicon dioxide are added, mixed evenly and pressed into tablets. The dissolution rate is 83.9%.
[0030] The unit dosage is 5 mg (calculated as nebivolol).
Embodiment 2
[0032] Nebivolol Hydrochloride 5.45g
[0033] PVPK30 21.8g
[0034] Lactose 72.75g
[0035] Croscarmellose Sodium 12.00g
[0036] Magnesium Stearate 1.00g
[0037] Silica 3.00g
[0038] 10% PVPK30 ethanol solution appropriate amount
[0039]
[0040] Make 1000 tablets
[0041] Use 10% PVPK30 ethanol solution to make lactose into soft material, pass through a 40-mesh sieve to granulate, dry at 50-60°C, and sieve through a 40-mesh sieve; put nebivolol hydrochloride and PVPK30 in a planetary ball mill, mix and grind at 500rpm for 15min. After the above materials are mixed evenly, 12% croscarmellose sodium, 1% magnesium stearate and 2.5% silicon dioxide are added, mixed evenly and pressed into tablets. The dissolution rate is 91.4%.
[0042] The unit dosage is 5 mg (calculated as nebivolol).
Embodiment 3
[0044] Nebivolol Hydrochloride 5.45g
[0045] PVPK30 27.25g
[0046] Lactose 67.3g
[0047] Croscarmellose Sodium 12.00g
[0048] Magnesium Stearate 1.00g
[0049] Silica 3.00g
[0050] 10% PVPK30 ethanol solution appropriate amount
[0051]
[0052] Make 1000 tablets
[0053]Use 10% PVPK30 ethanol solution to make lactose into soft material, pass through a 40-mesh sieve to granulate, dry at 50-60°C, and granulate with a 40-mesh sieve; put nebivolol hydrochloride and PVPK30 in a planetary ball mill, mix and grind at 500rpm for 15min. After the above materials are mixed evenly, 12% croscarmellose sodium, 1% magnesium stearate and 2.5% silicon dioxide are added, mixed evenly and pressed into tablets. The dissolution rate is 88.3%.
[0054] The unit dosage is 5 mg (calculated as nebivolol).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com