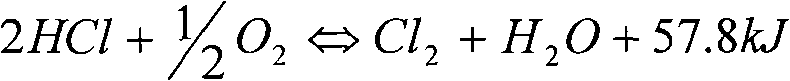Catalyst used for catalytic oxidation of hydrogen chloride for preparing chlorine gas and preparation method thereof
A catalytic oxidation and catalyst technology, applied in the direction of chloride preparation, physical/chemical process catalyst, chlorine/hydrogen chloride, etc., to achieve good stability, improve activity and stability, and simple and easy preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Use 56ml containing 25.33gCuCl 2 2H 2 O, 5gKCl and 7.57gCeCl 3 ·7H 2 The aqueous solution of O impregnated 70g of alumina carrier, then stirred at 40°C for 4h, stood at 30°C for 16h, dried at 100°C for 16h, and calcined at 400°C for 6h to prepare the copper chloride catalyst.
[0034] 30g cupric chloride catalyst is packed in the fixed-bed reactor, then hydrogen chloride gas and oxygen are introduced in the fixed-bed reactor with the feeding rate of 300ml / min and 150ml / min respectively, and reaction temperature is 365 ℃, reaction pressure 0.2Mpa. After 4 hours of reaction, the conversion rate of hydrogen chloride was 86.3%, and after 100 hours of reaction, the conversion rate of hydrogen chloride was 86.0%, and the activity of the catalyst remained basically unchanged.
Embodiment 2
[0036] Use 64ml containing 12.67gCuCl 2 2H 2 O, 5gKCl and 7.56gLaCl 3 ·nH 2 The aqueous solution of O impregnated 80g of alumina carrier, then stirred at 50°C for 3h, stood at 40°C for 12h, dried at 110°C for 12h, and calcined at 500°C for 4h to prepare the copper chloride catalyst.
[0037] The processing condition of catalytic reaction is identical with embodiment 1. After 4 hours of reaction, the conversion rate of hydrogen chloride was 84.8%, and after 100 hours of reaction, the conversion rate of hydrogen chloride was 84%, and the activity of the catalyst remained basically unchanged.
Embodiment 3
[0039] Use 60ml containing 12.67gCuCl 2 2H 2 O, 5gKCl and 15.09gPrCl 3 ·7H 2 The aqueous solution of O impregnated 75g of alumina carrier, then stirred at 60°C for 2h, stood at 50°C for 8h, dried at 120°C for 8h, and calcined at 600°C for 4h to prepare the copper chloride catalyst.
[0040] The processing condition of catalytic reaction is identical with embodiment 1. After 4 hours of reaction, the conversion rate of hydrogen chloride was 85.8%, and after 100 hours of reaction, the conversion rate of hydrogen chloride was 85.6%, and the activity of the catalyst remained basically unchanged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com