Composition of secnidazole and lactose and preparation method thereof
A technology of secnidazole and composition, which is applied in the field of secnidazole and lactose composition and preparation thereof, and achieves the effects of good clinical effect, good disintegration effect and compressibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
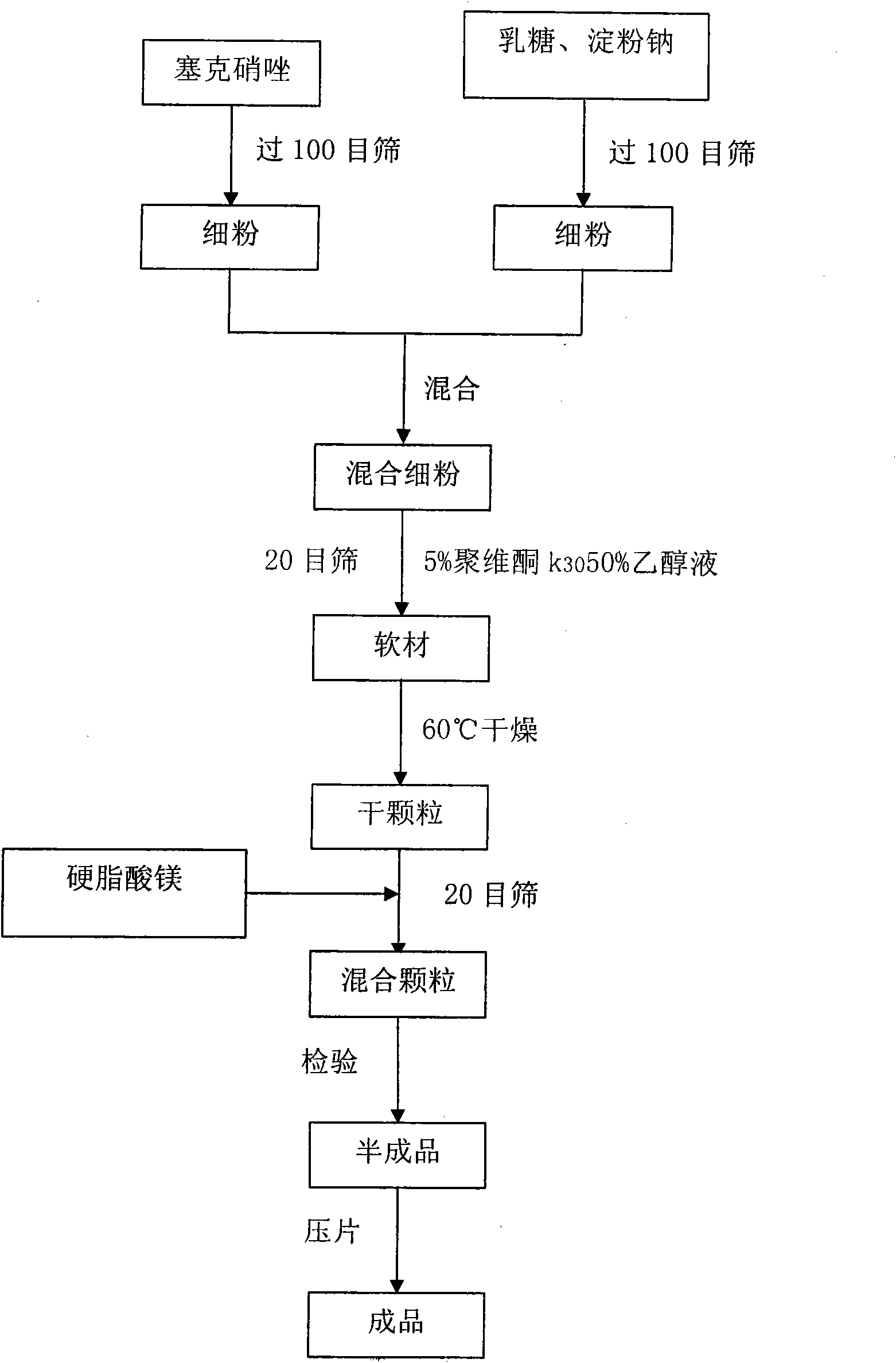
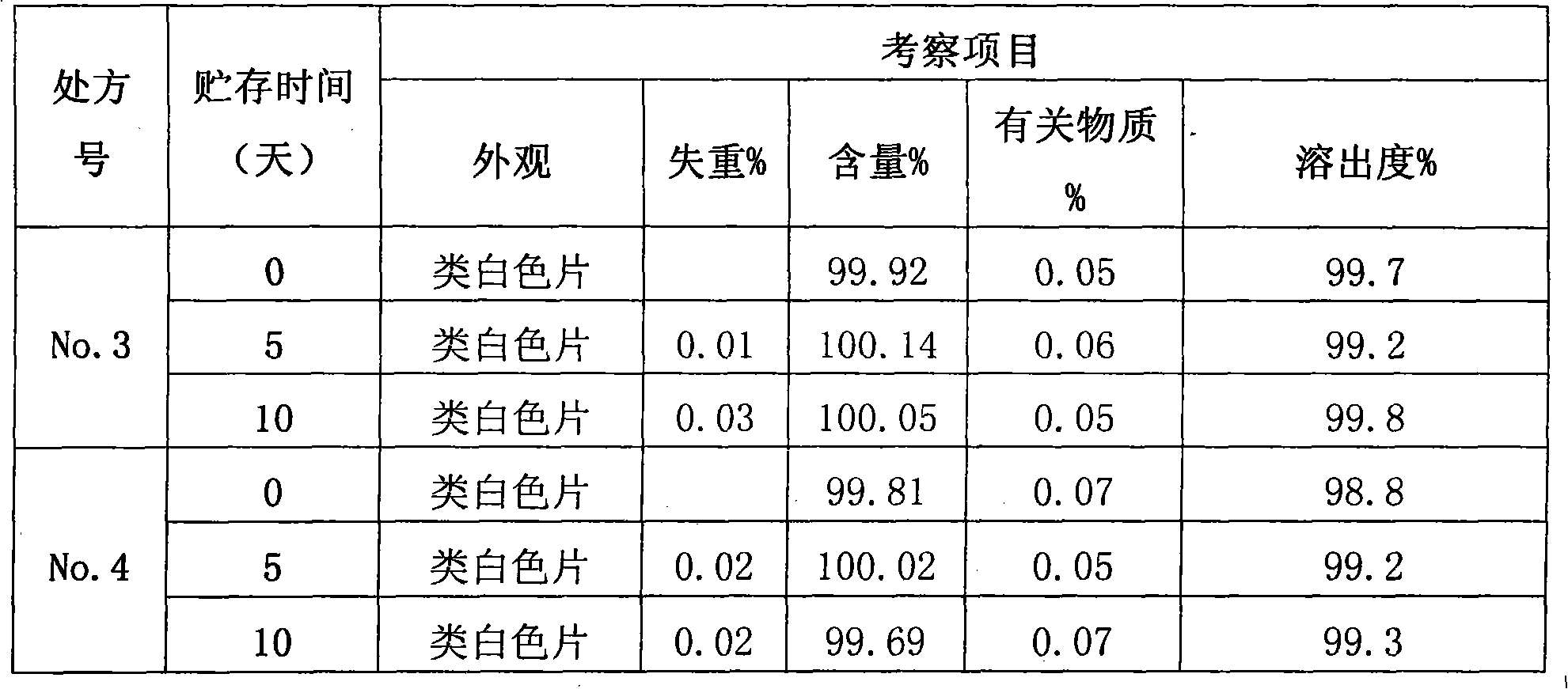
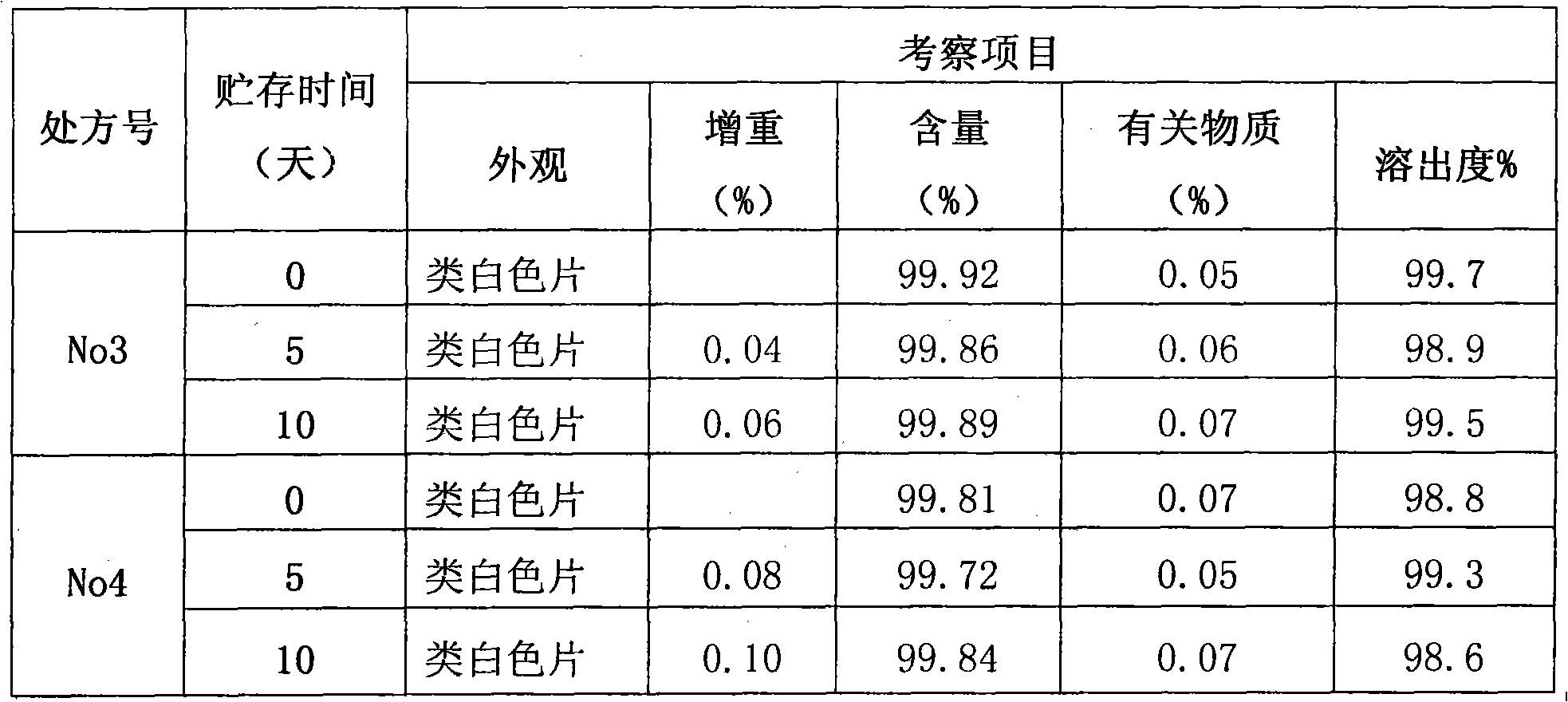
[0024] See attached figure 1 , the present invention relates to a kind of secnidazole and lactose composition, it is characterized in that, said composition has: secnidazole 500g, lactose 76g, carboxymethyl starch sodium 24g, povidone ethanol liquid Appropriate amount, magnesium stearate 2.8g.
[0025] In addition, if the tablet is made into 50 tablets, it will contain: 25.0 g of secnidazole, 4.2 g of lactose, 0.8 g of sodium starch glycolate, an appropriate amount of ethanol solution of povidone, and 0.13 g of magnesium stearate.
[0026] In addition, if the tablet is made into 50 tablets, it will contain: 25.0 g of secnidazole, 4.0 g of lactose, 1.0 g of sodium starch glycolate, an appropriate amount of povidone ethanol solution, and 0.14 g of magnesium stearate.
[0027] In addition, if the tablet is made into 50 tablets, it will contain: 25.0 g of secnidazole, 3.8 g of lactose, 1.2 g of sodium starch glycolate, an appropriate amount of povidone ethanol solution, and 0.14 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com