Method for bionically preparing water-soluble gold nanoparticles
A gold nanoparticle and water-soluble technology, which is applied in the fields of chemistry and materials science, can solve the problems of reaction mechanism research, lack of clarity, and difficult control of product morphology, and achieve the effect of mild conditions and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
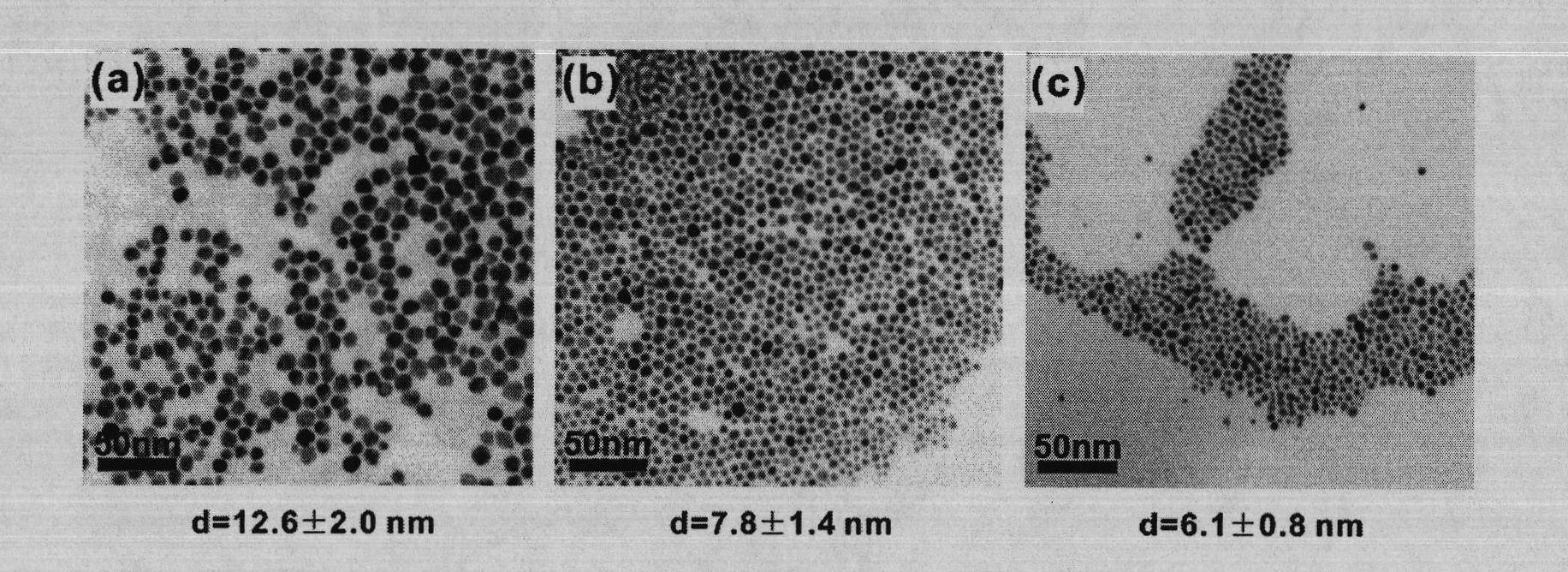
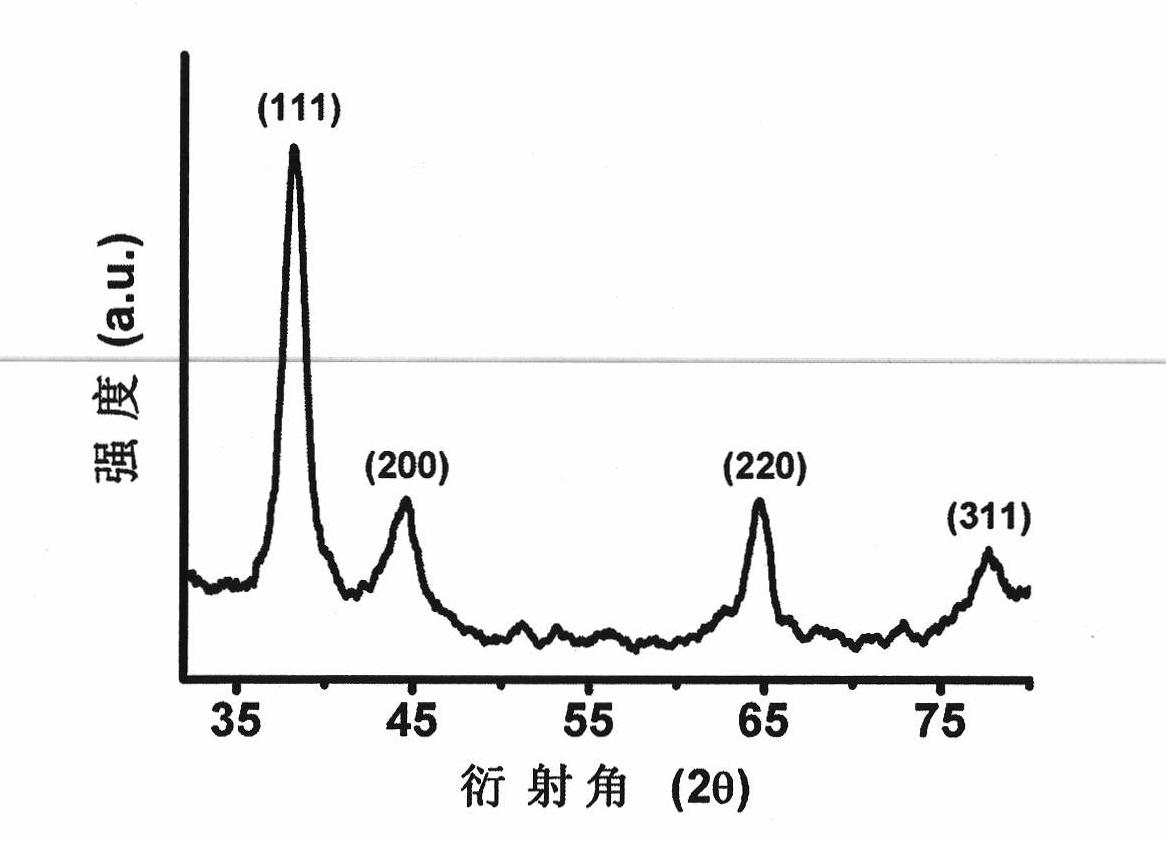
[0019] The preparation of embodiment 1 gold nanoparticles
[0020] (1) 1g chloroauric acid is dissolved in 100mL ultrapure water, is made into the chloroauric acid stock solution of 1% (weight to volume ratio);
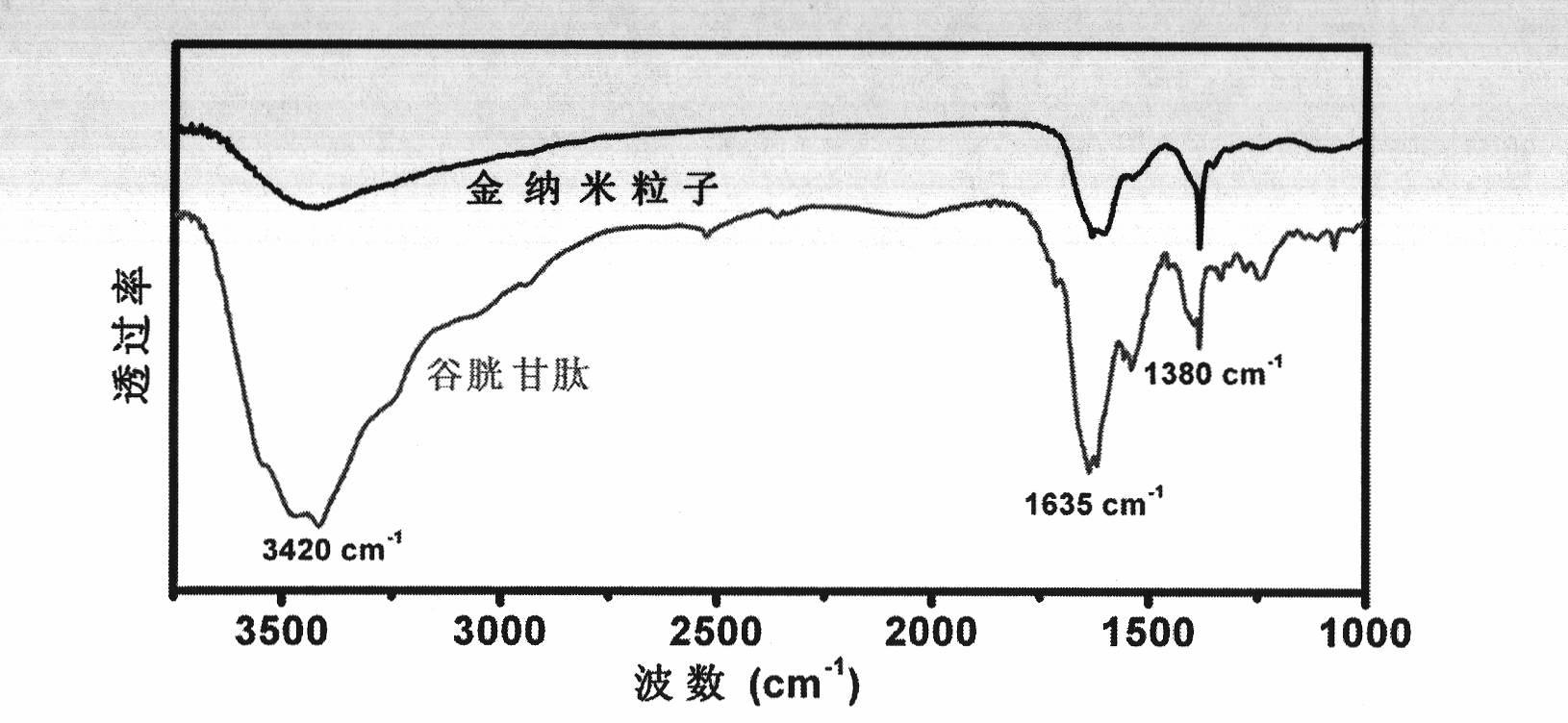
[0021] (2) chloroauric acid solution is mixed with reduced glutathione (GSH) in a molar ratio of 1:1;
[0022] (3) The pH value of the reaction solution is adjusted to 2.5-3.0 with sodium hydroxide solution to obtain a pale yellow precipitate;
[0023] (4) centrifugation (RCF 10000g), the precipitate is dissolved with 0.01mol / L sodium hydroxide solution to obtain a gold precursor solution;
[0024] (5) Dilute the gold precursor solution to 0.64 mmol / L with ultrapure water, add NADPH and GR at a ratio of 2 mg NADPH and 1 unit GR per ml of the diluted solution, and stir for 12 hours at room temperature at 25°C;
[0025] (6) The reaction solution was ultrafiltered with an ultrafiltration tube (Millipore) with a molecular weight cut-off of 30 kDa, and components with ...
Embodiment 2
[0026] The preparation of embodiment 2 gold nanoparticles
[0027] (1) 1g chloroauric acid is dissolved in 100mL ultrapure water, is made into 1% chloroauric acid stock solution;
[0028] (2) chloroauric acid solution is mixed with reduced glutathione (GSH) in a molar ratio of 1:1;
[0029] (3) The pH value of the reaction solution is adjusted to 2.5-3.0 with sodium hydroxide solution to obtain a pale yellow precipitate;
[0030] (4) Centrifuge (RCF 10000g) for 3 minutes, and dissolve the precipitate with 35ml of 0.01mol / L sodium hydroxide solution to obtain a gold precursor solution;
[0031] (5) Dilute the gold precursor solution to 0.64mmol / L with ultrapure water, add NADPH and GR at a ratio of 3mg NADPH and 1 unit GR per milliliter of the diluted solution, and stir for 12 hours at room temperature at 25°C;
[0032] (6) The reaction solution was ultrafiltered with an ultrafiltration tube (Millipore) with a molecular weight cut-off of 30 kDa, and components with a molecu...
Embodiment 3
[0033] The preparation of embodiment 3 gold nanoparticles
[0034] (1) 1g chloroauric acid is dissolved in 100mL ultrapure water, is made into 1% chloroauric acid stock solution;
[0035] (2) chloroauric acid solution is mixed with reduced glutathione (GSH) in a molar ratio of 1:1;
[0036] (3) The pH value of the reaction solution is adjusted to 2.5-3.0 with sodium hydroxide solution to obtain a pale yellow precipitate;
[0037] (4) Centrifuge (RCF 10000g) for 3 minutes, and dissolve the precipitate with 35ml of 0.01mol / L sodium hydroxide solution to obtain a gold precursor solution;
[0038] (5) Dilute the gold precursor solution to 0.64 mmol / L with ultrapure water, add NADPH and GR at a ratio of 4 mg NADPH and 1 unit GR per ml of the diluted solution, and stir and react at room temperature 25°C for 12 hours;
[0039] (6) The reaction solution was ultrafiltered with an ultrafiltration tube (Millipore) with a molecular weight cut-off of 30 kDa, and components with a molec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com