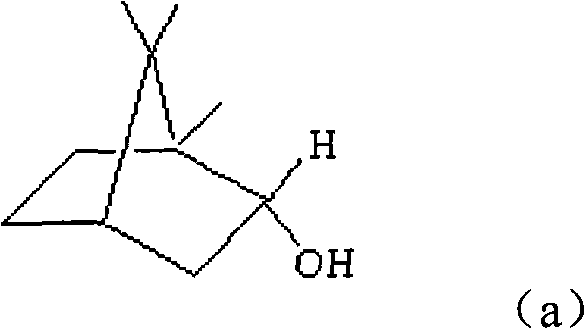
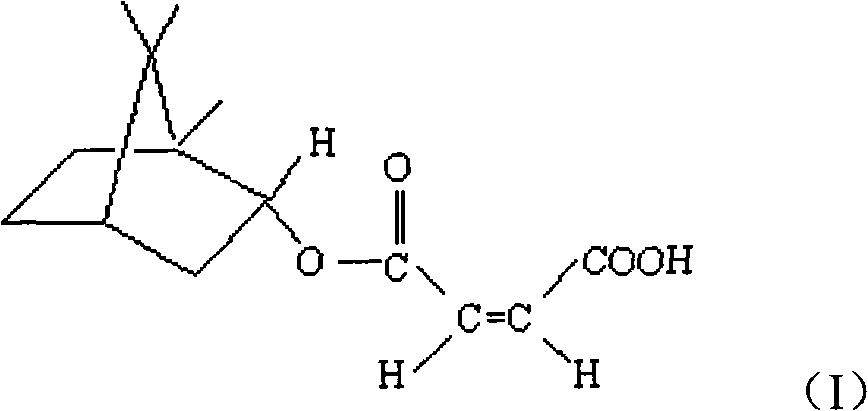
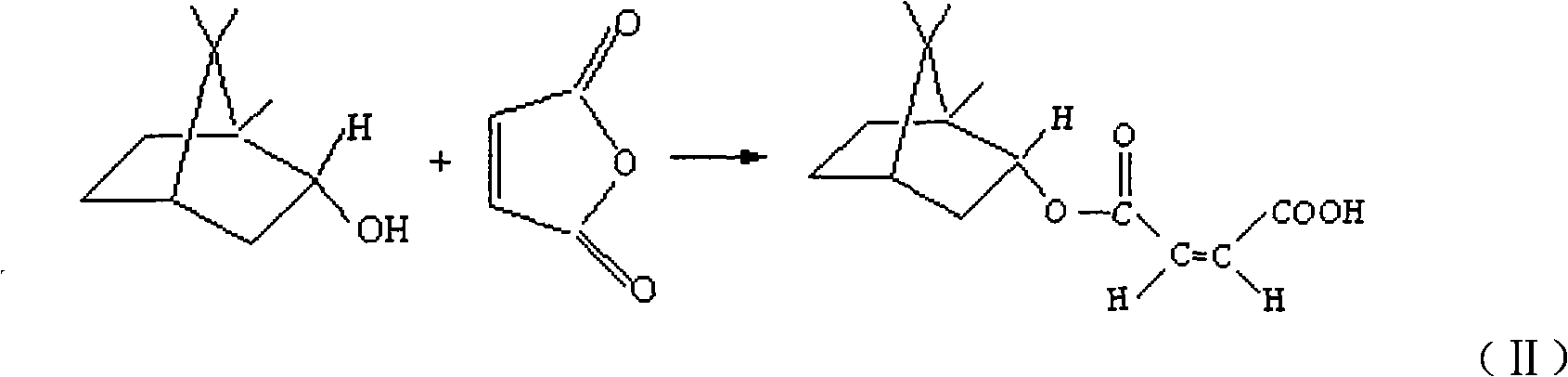
(+)-monobornyl maleinate as well as preparation method and application thereof
A technology of maleic acid mono- and maleic anhydride, which is applied in the chemical field and can solve the problems of easy volatilization, nasal mucosal toxicity, and poor water solubility of borneol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] Weigh 7 g of maleic anhydride and 8 g of natural (+)-borneol and place in a mortar, mix and grind and place in a round bottom flask equipped with a magnetic stirrer, reflux tube and drying tube, in an oil bath at 80 °C Heat to dissolve, stir and reflux for 7 hours, pour the reaction solution into a 250 ml separatory funnel, wash with distilled water three times (the amount of distilled water for each wash is 20ml, 20ml and 10ml respectively), separate the oil layer for subsequent use, and wash the water layer with ethyl acetate The ester was extracted twice (the amount of ethyl acetate used for each extraction was 10ml), and the ethyl acetate layer was taken, combined with the above oil layer, and dried over anhydrous magnesium sulfate. After drying, use silica gel column chromatography with a silica gel particle size of 200 to 300 meshes, elute with ethyl acetate-petroleum ether at a volume ratio of 0.1:6, and at the same time, use ethyl acetate-n-hexane with a volume r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com