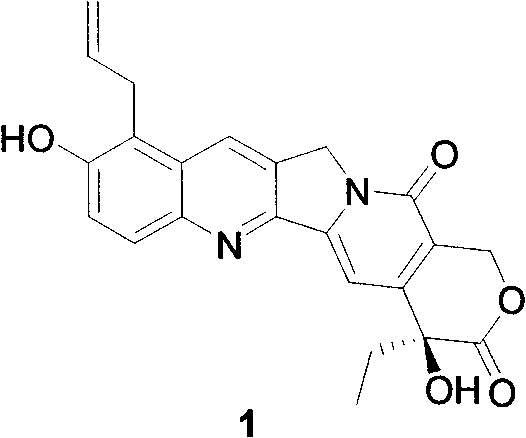
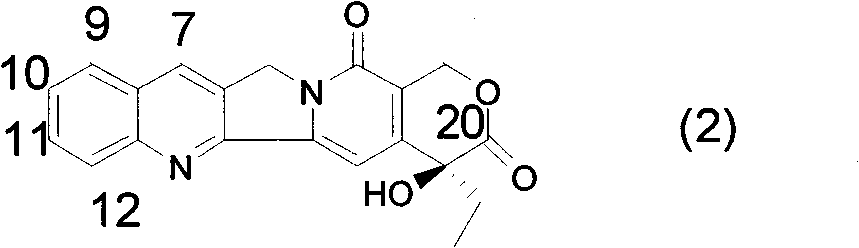
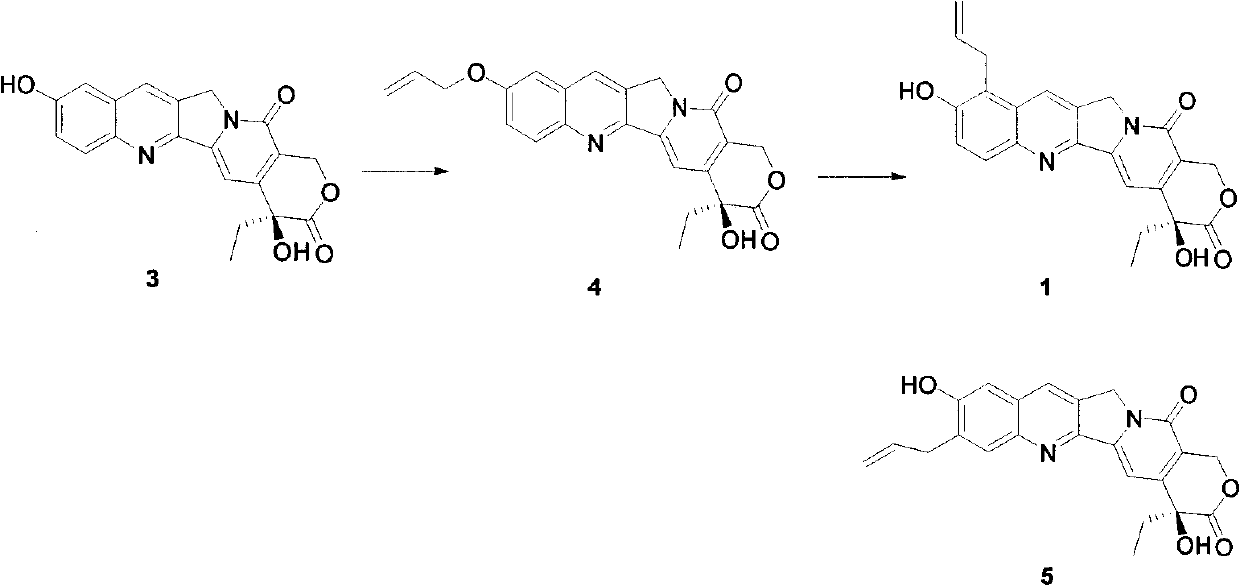
Method for synthetizing allyl-substituted camptothecin compound
A technology of hydroxycamptothecin and allyl, which is applied in the field of drug synthesis and can solve problems such as highly toxic tin reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0026] 9-iodo-10-hydroxycamptothecin:
[0027] 10-Hydroxycamptothecin (2.0g, 5.5mmol) was dissolved in DMF (50ml), cooled to 0°C in an ice-water bath, N-iodosuccinimide (1.24g, 5.5mmol) was added, and reacted at room temperature for 2h After the reaction is completed, pour it into 200ml of ice water and adjust the pH value to 3-4 with 1N HCl. After fully stirring, filter with suction, wash with water, and dry to obtain 2.5 g of a yellow solid with a yield of 95%. 1 HNMR (DMSO-d 6 )(ppm) 11.2(1H, s), 8.72(1H, s), 8.07(1H, d), 7.63(1H, d), 7.28(1H, s), 6.50(1H, s), 5.42(2H, s), 5.29 (2H, s), 1.83-1.90 (2H, m), 0.88 (3H, t).
[0028] 9-iodo-10-methoxymethylcamptothecin:
[0029] Disperse 2.0g of 9-iodo-10-hydroxycamptothecin in 200ml of anhydrous dichloromethane, add 1.4ml of diisopropylethylamine, slowly add 0.4ml of chloromethyl ether to the system at room temperature, and Continue the reaction at room temperature for 2 hours. After the reaction is completed, pour it into ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com