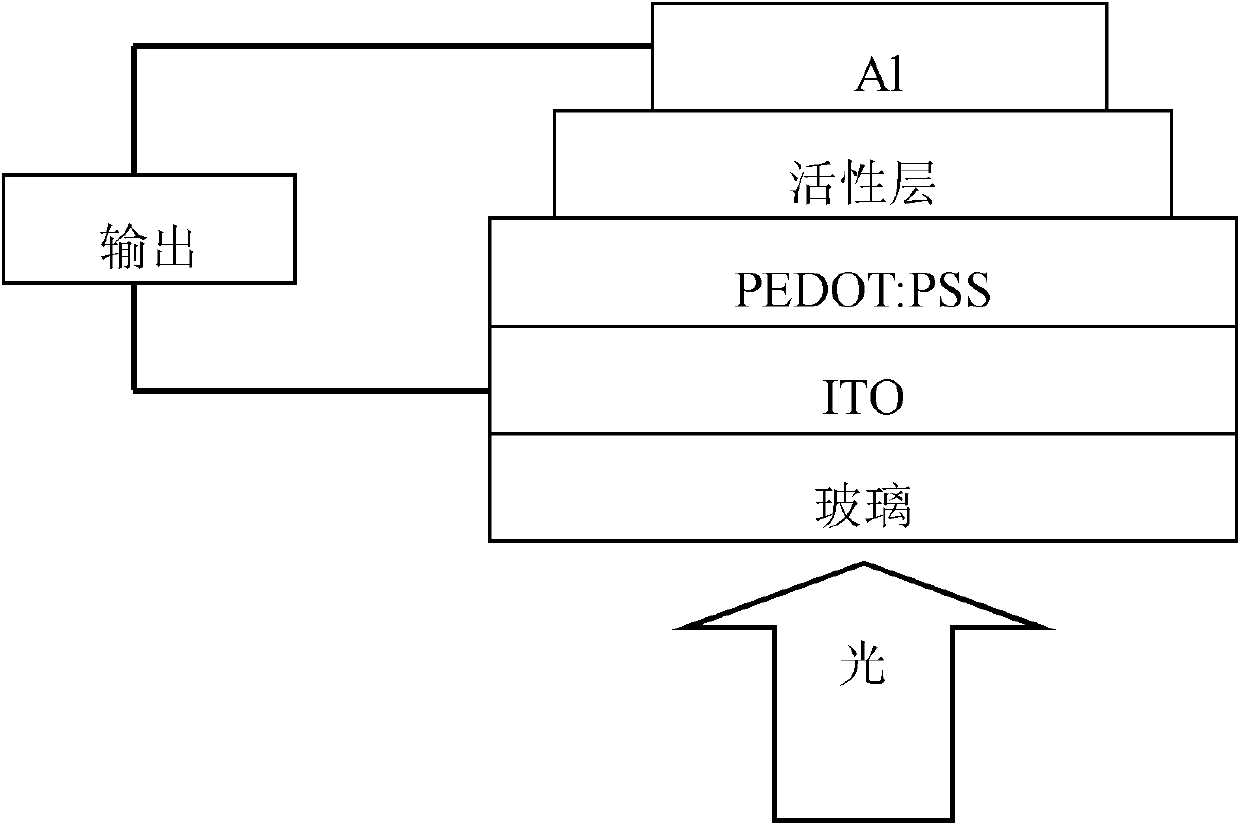
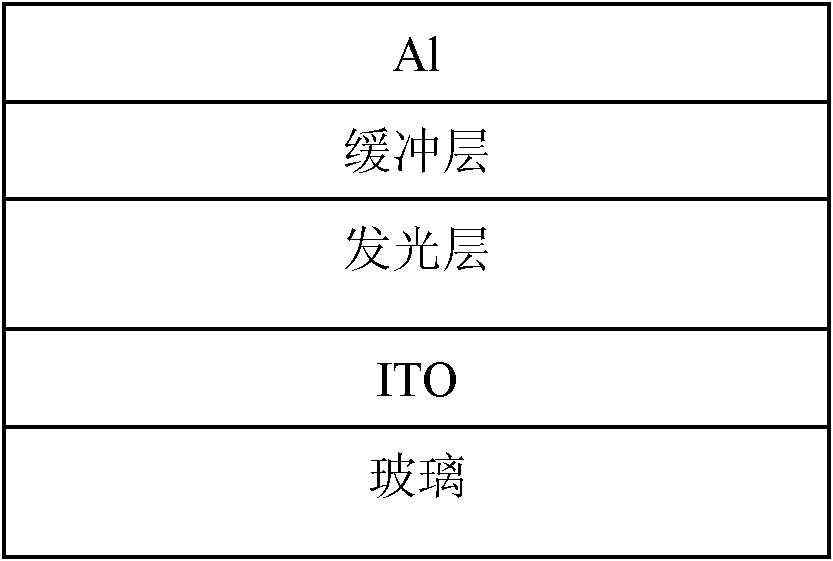
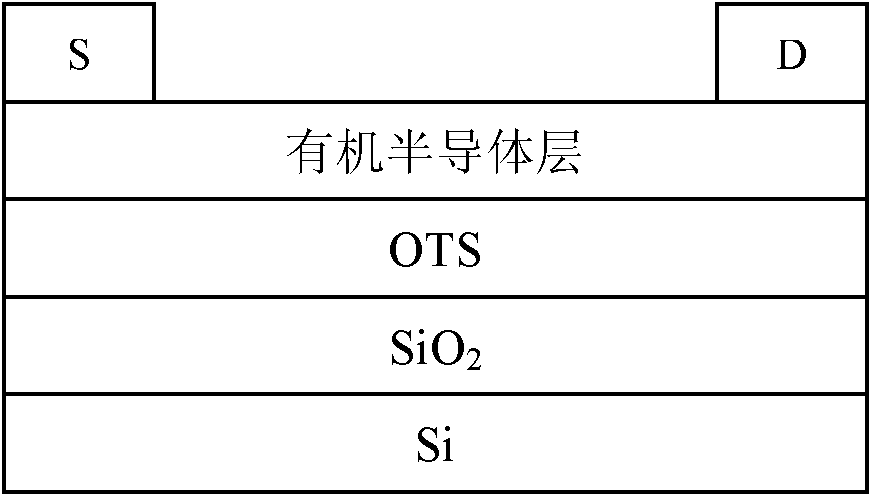
Fluorene copolymer containing thiophene and pyrrolopyrrole units, preparation method and application thereof
A diketopyrrolopyrrole copolymer technology, which is applied in the field of preparation of fluorene copolymers containing thiophene and pyrrolopyrrole units, can solve the problems of solar radiation spectrum mismatch and low photoelectric conversion efficiency of organic solar cells, and achieve Improved stability, improved solubility and processability, and high yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] Preparation of fluorene monomers
[0034] Fluorene monomer B 1 The preparation method is shown in the following formula:
[0035]
[0036] In the formula, R 1 and R 2 Same or different as H or C 1 ~C 20 of alkyl.
[0037] At a temperature of -100 to -25°C, 2,7-dibromo-9,9-dialkylfluorene A 1 Add n-butyllithium to the organic solvent in a molar ratio of 1.0:2.0~4.0, then add 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane , and its dosage is calculated as A in terms of moles 1 2.0 to 4.0 times of that, reacted for 24 to 48 hours to obtain product B1, namely 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)- 9,9-Dialkylfluorenes. The organic solvent used in this reaction is preferably tetrahydrofuran, diethyl ether, dichloromethane, chloroform or ethyl acetate and the like.
[0038] Preparation of thiophene monomers
[0039] Thiophene monomer B 2 The preparation is shown in the following formula:
[0040]
[0041] In the formula, R 9 , R 10 same or...
Embodiment 1
[0059] Embodiment 1, the fluorene copolymer containing thiophene and diketopyrrolopyrrole units, its chemical formula is as follows:
[0060]
[0061] In the formula, x=0.5, y=0.5, n=27.
[0062] One, preparation of fluorene monomer 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dimethylfluorene, which The chemical formula is as follows:
[0063]
[0064] Under the condition of -100℃ and nitrogen protection, add 20.00mL of n-butyllithium solution with a concentration of 1.00M to a reaction flask containing 3.52g of 2,7-dibromo-9,9-dimethylfluorene and 100mL of tetrahydrofuran After stirring for 2 hours, slowly add 4.17mL 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane dropwise, return to room temperature and continue stirring React for 24 hours. After the reaction was completed, the reaction solution was poured into water, extracted with ether, dried over anhydrous magnesium sulfate, removed by rotary evaporation and column chromatography to obtain a solid p...
Embodiment 2
[0088] Embodiment 2, the fluorene copolymer containing thiophene and diketopyrrolopyrrole units, its chemical formula is as follows:
[0089]
[0090] In the formula, x=0.5, y=0.5 n=41.
[0091] One, preparation of fluorene monomer 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dimethylfluorene, which The chemical formula is as follows:
[0092]
[0093] The preparation steps are detailed in Example 1.
[0094] Two, prepare thiophene monomer 2,5-dibromo-3,6-dimethylthieno[3,2-b]thiophene, its chemical formula is as follows:
[0095]
[0096] ① Preparation of thiophene raw material 3,6-dimethylthieno[3,2-b]thiophene, its chemical formula is as follows:
[0097]
[0098] Add 12.00 g of 3,6-dibromo-thieno[3,2-b]thiophene and 132 mg (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) chloride into the Place in a 100mL vial with a stir bar, seal it, and flush it with nitrogen. Then 30 mL of tetrahydrofuran and 50 mL of tetrahydrofuran solution containing 1.0 M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com