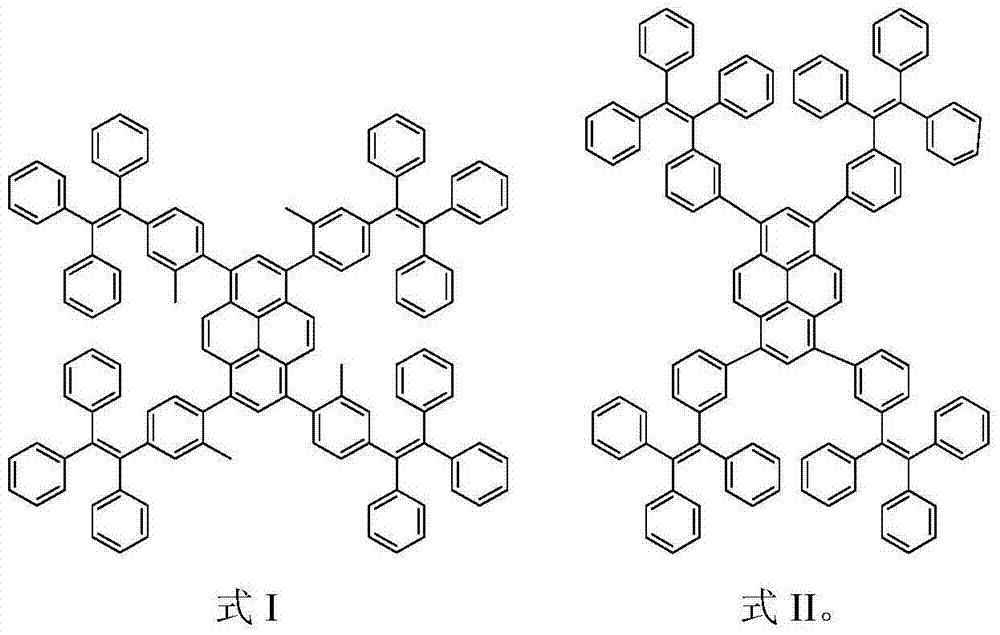
Aggregation-induced light-emitting molecule based on tetraphenylethylenes and preparation method and use thereof
A technology of aggregation-induced luminescence and tetraphenylethylene, which is applied in the fields of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., to achieve the effects of high efficiency, simple preparation, and increased transmission capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
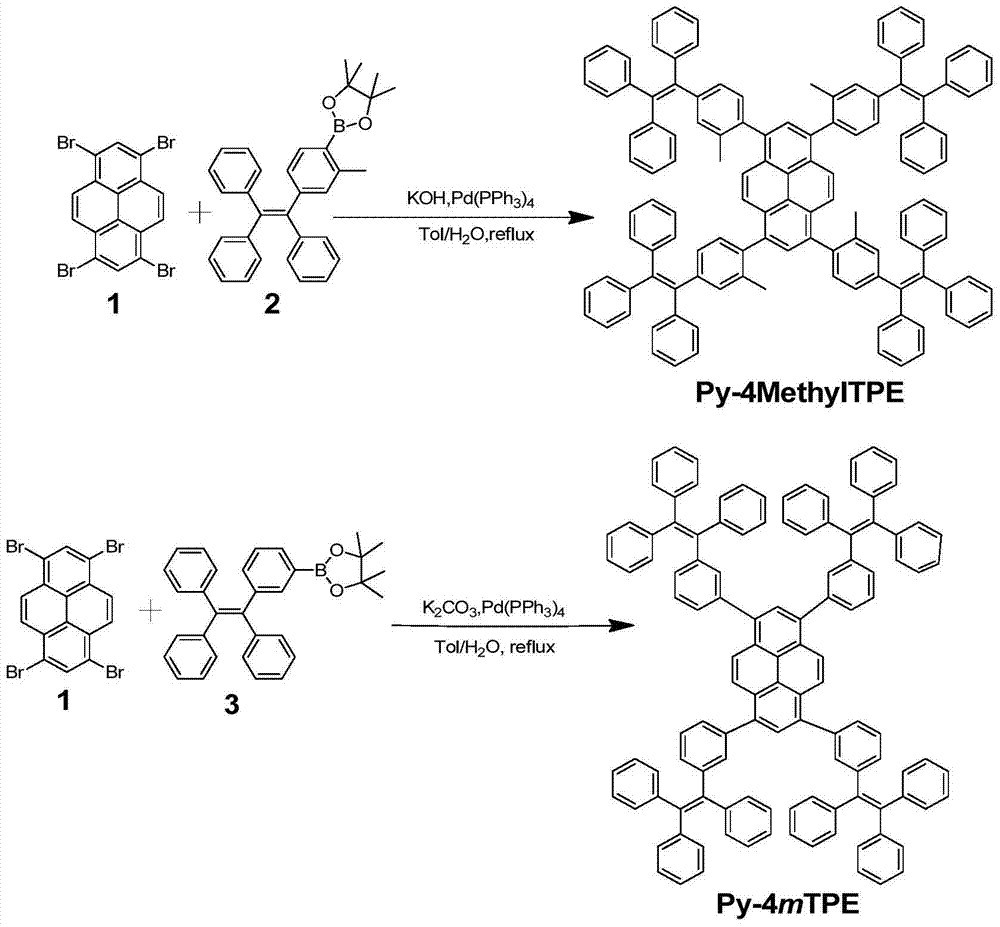
[0027] The synthesis of embodiment 1Py-4MethylTPE
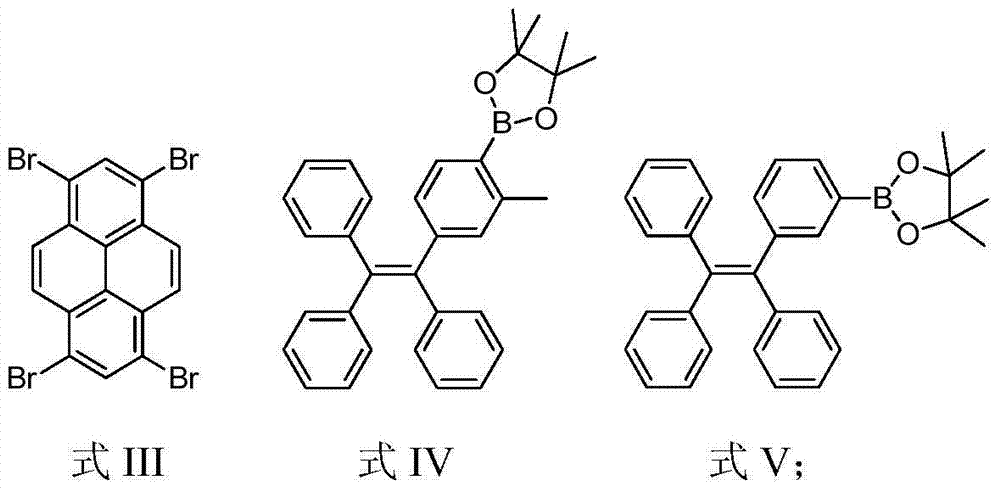
[0028] Under nitrogen protection, compound 1 (260 mg, 0.5 mmol) and compound 2 (1.166 g, 2.47 mmol), potassium hydroxide (560 mg, 10 mmol) and Pd(PPh 3 ) 4 (0.20g, 0.08mmol), then add 30mL toluene and 10mL deoxygenated water, and reflux at 80°C for 3 days to fully react. After the reaction was completed, the product was cooled to room temperature, then extracted with chloroform, the organic phase was collected, and then extracted with anhydrous Na 2 SO 4 Dried and spin-dried to obtain a crude product. Using petroleum ether and chloroform (v / v, 1 / 1) as eluent, the product is carried out preliminary separation and purification with silica gel column chromatography, and finally dichloromethane and petroleum ether (v / v, 1 / 1 10) reprecipitate to obtain a white solid (230mg, yield 29%), and use 1 The structure was characterized by H NMR and MS, which confirmed that the white solid was the compound Py-4MethylTPE. 1 H NMR (300M...
Embodiment 2
[0029] The synthesis of embodiment 2Py-4mTPE
[0030] Under nitrogen protection, compound 1 (260 mg, 0.5 mmol) and compound 3 (940 mg, 2.05 mmol), potassium carbonate (1.38 g, 10 mmol) and Pd(PPh 3 ) 4 (0.20 g, 8% mmol), and then add 30 mL of toluene and 10 mL of deoxygenated water, and reflux at 80° C. for 3 days to fully react. After the reaction was completed, the product was cooled to room temperature, then extracted with chloroform, the organic phase was collected, and then extracted with anhydrous Na 2 SO 4 Dried and spin-dried to obtain a crude product. Using sherwood oil and chloroform (v / v, 1 / 1) as eluent, the product is initially separated and purified by silica gel column chromatography, and finally it is washed with dichloromethane and sherwood oil (v / v, 1 / 10) was reprecipitated to obtain a yellow solid (350mg, yield 46%), and 1 H NMR, 13 The structure was characterized by C NMR, MS and EA, which confirmed that the yellow solid was the compound Py-4mTPE. 1 H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com