Aggregation-induced luminescence molecules based on tetraphenylethylene, preparation method and application thereof
A technology of aggregation-induced luminescence and tetraphenylethylene, which is applied in the fields of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., to achieve the effects of increasing transmission capacity, increasing thermal stability, and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
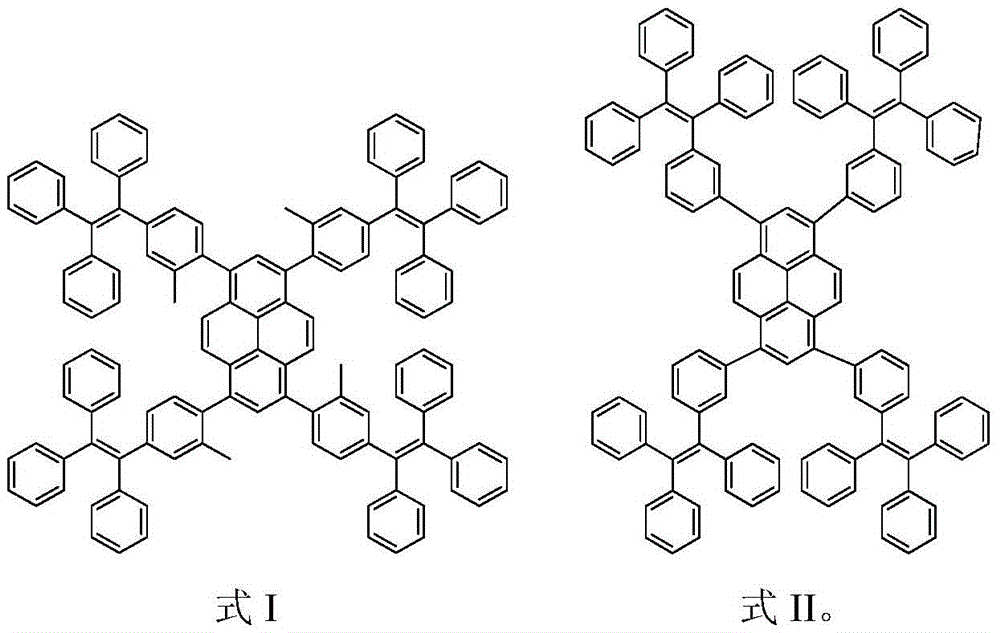
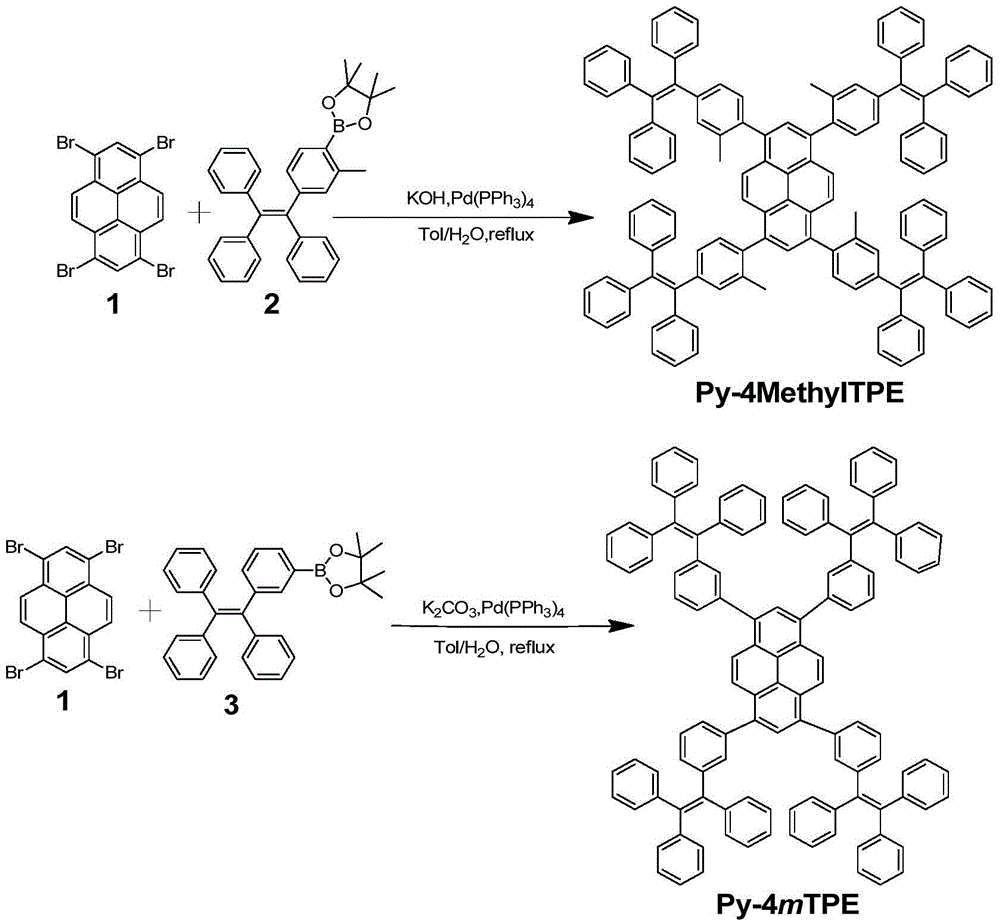
[0027] Example 1 Synthesis of Py-4MethylTPE
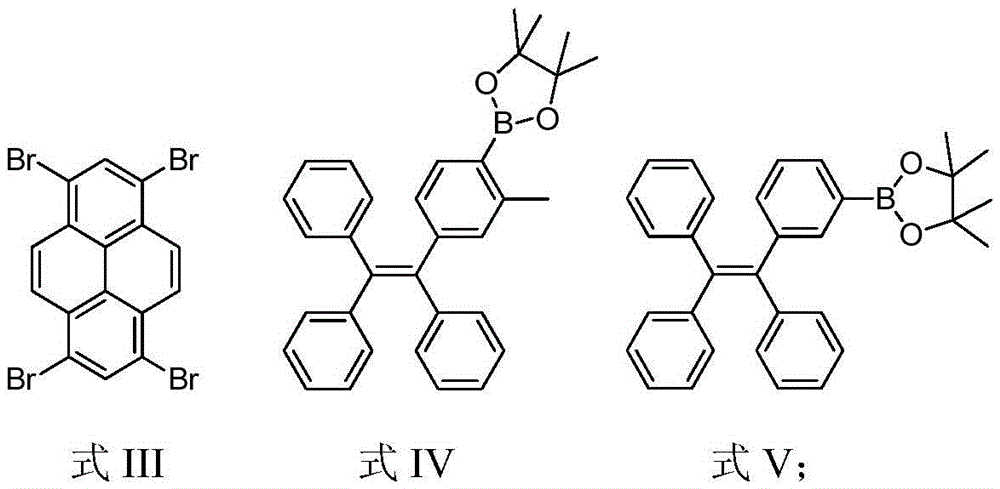
[0028] Under the protection of nitrogen, add compound 1 (260mg, 0.5mmol) and compound 2 (1.166g, 2.47mmol), potassium hydroxide (560mg, 10mmol) and Pd(PPh) to the Schlenk tube 3 ) 4 (0.20g, 0.08mmol), then add 30mL toluene and 10mL deoxygenated water, reflux at 80°C for 3 days for full reaction. After the reaction is complete, cool the product to room temperature, then extract with chloroform, collect the organic phase, and then use anhydrous Na 2 SO 4 Dry and spin dry to obtain a crude product. Using petroleum ether and chloroform (v / v, 1 / 1) as eluents, the product was preliminarily separated and purified by silica gel column chromatography, and finally dichloromethane and petroleum ether (v / v, 1 / 10) Perform reprecipitation to obtain a white solid (230mg, yield 29%), and use 1 The structure was characterized by HNMR and MS, and it was confirmed that the white solid was compound Py-4MethylTPE. 1 HNMR(300MHz, CDCl 3 δ): 7.70 (s, 2H), ...
Embodiment 2
[0029] Example 2 Synthesis of Py-4mTPE
[0030] Under the protection of nitrogen, add compound 1 (260mg, 0.5mmol) and compound 3 (940mg, 2.05mmol), potassium carbonate (1.38g, 10mmol) and Pd(PPh) to the Schlenk tube 3 ) 4 (0.20 g, 8% mmol), then add 30 mL of toluene and 10 mL of deoxygenated water, and reflux at 80° C. for 3 days to fully react. After the reaction is complete, cool the product to room temperature, then extract with chloroform, collect the organic phase, and then use anhydrous Na 2 SO 4 Dry and spin dry to obtain a crude product. Using petroleum ether and chloroform (v / v, 1 / 1) as eluents, the product was preliminarily separated and purified by silica gel chromatography, and finally it was purified with dichloromethane and petroleum ether (v / v, 1 / 10) reprecipitation to obtain a yellow solid (350mg, yield 46%), and use 1 HNMR, 13 The structure was characterized by CNMR, MS and EA, and it was confirmed that the yellow solid was compound Py-4mTPE. 1 HNMR (300MHz, CDCl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com