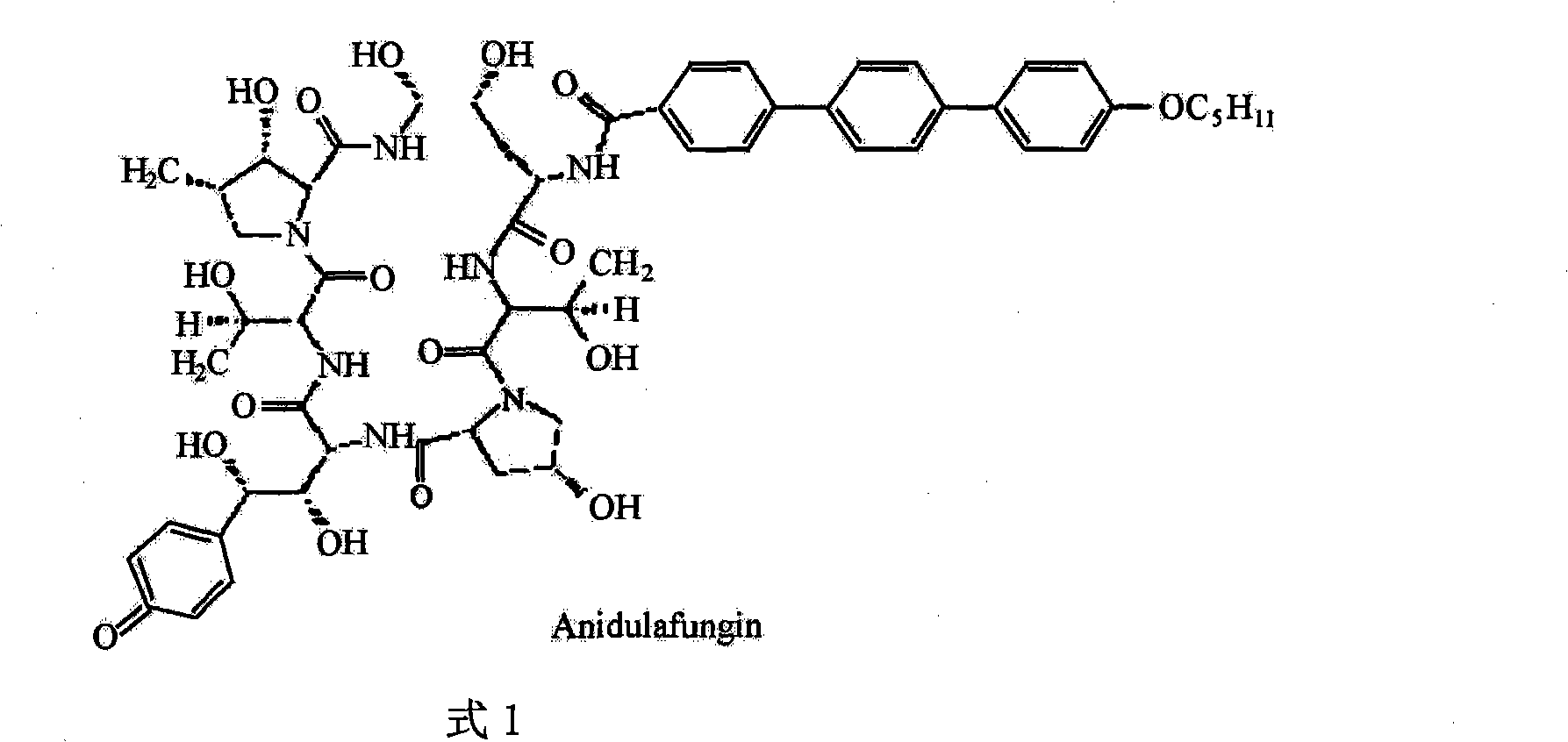
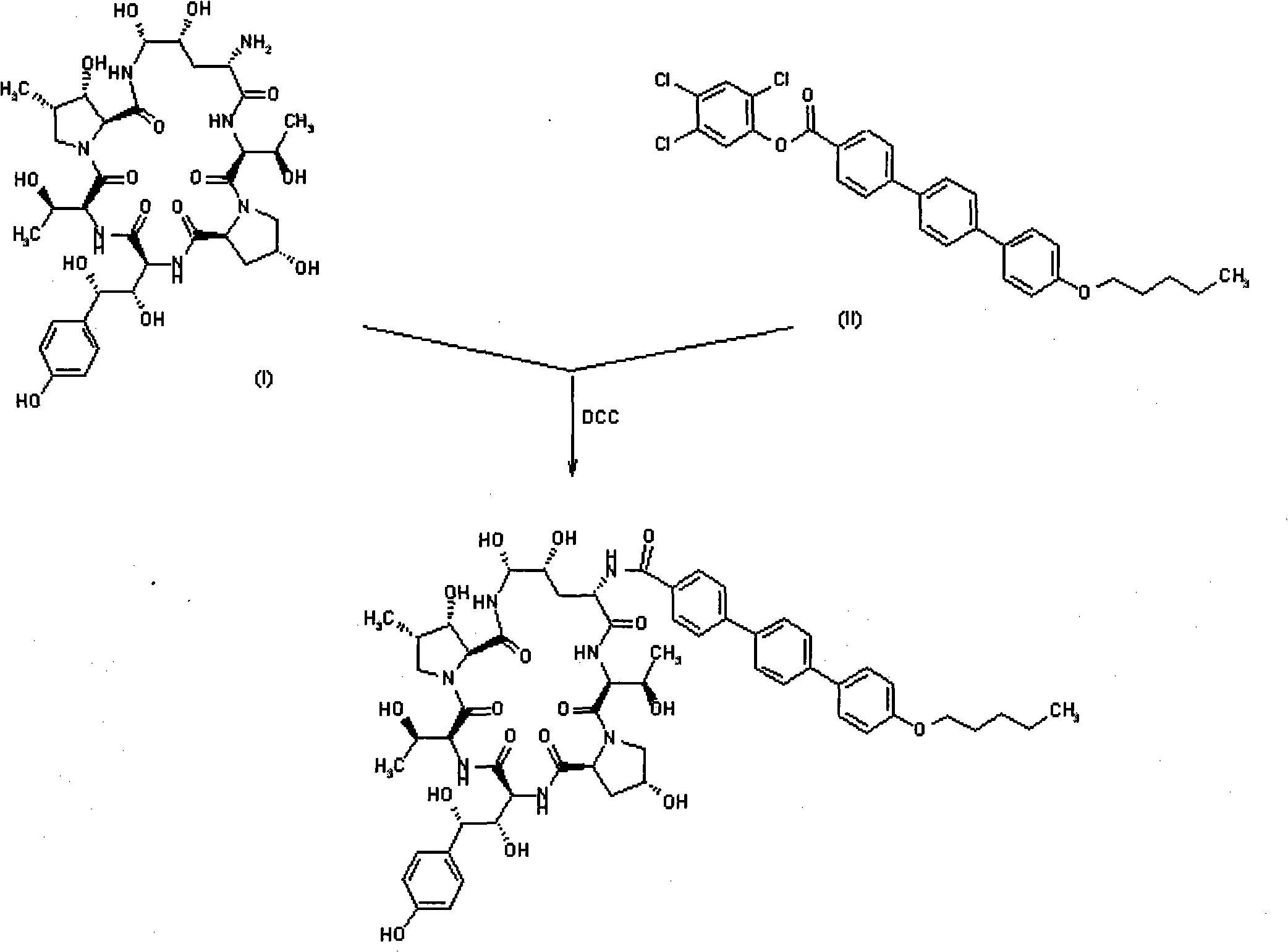
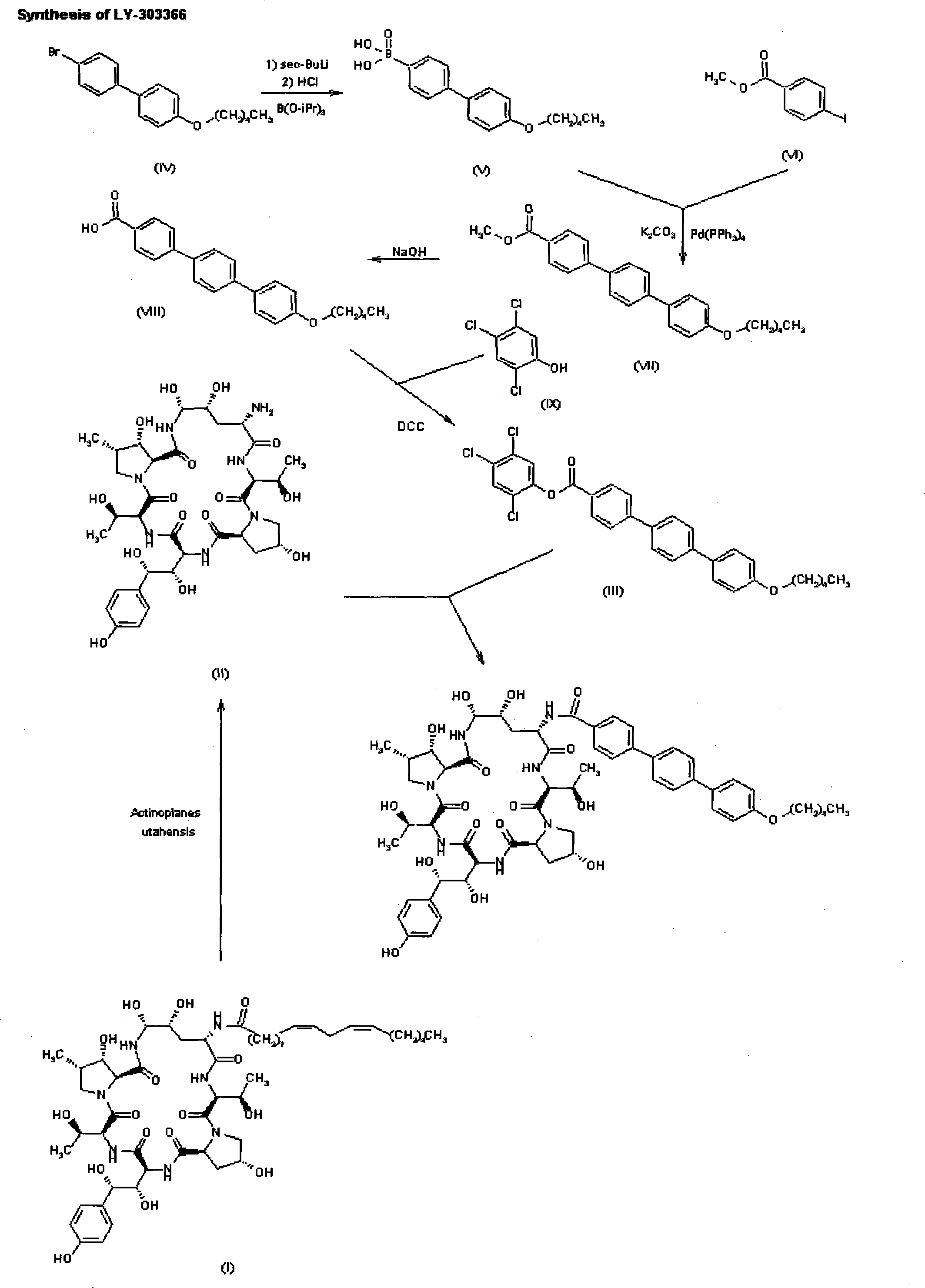
Preparation method of anidulafungin side chain intermediate
A technology of anidulafungin and an intermediate, which is applied in the field of preparation of anidulafungin side chain intermediates, can solve the problems of uncontrollable reaction conditions, high price, difficult availability, etc., and achieves shortened hydrolysis reaction time, low cost, and low cost. Easy to use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Synthesis of 1,4-benzenediboronic acid
[0041] Under the protection of nitrogen, stirring add 220ml THF, 23.52g (0.98mol) magnesium, 1.0g (0.004mol) I 2 , Slowly add dropwise 315ml THF solution containing 68.4g (0.29mol) 1,4-dibromobenzene, after the dropwise addition, react at 35°C for 3h, cool to -75°C, slowly dropwise add trimethyl borate (123.2ml, 1.13) mol) and 300ml THF mixture, after dripping, react at -75°C for 1.5h, slowly rise to room temperature and stir for 15h. 980ml of 2.5M HCl was added and quickly stirred for 15min, filtered, the filter cake was washed three times with 320ml of n-hexane, and dried in vacuum to obtain 39.7g of white solid with a yield of 82.4%.
Embodiment 2
[0042] Example 2 Synthesis of 1,4-benzenediboronic acid
[0043] Under the protection of nitrogen, add 200ml THF, 15.0g (0.62mol) magnesium, 1.0g (0.004mol) I with stirring 2 , Slowly drop in 300ml THF solution containing 68.4g (0.29mol) 1,4-dibromobenzene, react at 30°C for 3h, cool to -70°C, slowly add trimethyl borate (78.5ml, 0.72 mol) and 300ml THF mixture, after dripping, react at -70°C for 1h, slowly rise to room temperature and stir for 12h. 950ml of 2.5M HCl was added and quickly stirred for 15min, filtered, the filter cake was washed three times with 300ml of n-hexane, and dried under vacuum to obtain 40.7g of white solid with a yield of 84.6%.
Embodiment 3
[0044] Example 3 Synthesis of 4″-n-pentyloxy-1,1′:4′,1″-terphenyl-4-carboxylic acid ethyl ester
[0045] Take 20.0g (120.6mmol) of 1,4-benzenediboronic acid and 13.6g (56.0mmol) of 4-pentyloxy bromobenzene, dissolve in 200ml of dioxane-ethanol (4:1) mixed solvent and 72ml (144.0) mmol) 2M Na 2 CO 3 Solution. The reaction solution was sonicated for 5 minutes and passed N 2 After removing oxygen, add 5.08g (6.99mmol) pd(dppf)cl 2 , In N 2 The reaction was heated under reflux for 5.2h under protection, 25ml of dioxane-ethanol (8:1) solvent containing 11.5g (50.4mmol) of ethyl 4-bromobenzoate was added dropwise, and the reaction was continued under reflux for 4h. Cool, filter, and wash the filter cake with 55ml toluene, 53ml methyl tert-butyl ether, 50ml water, 18ml methyl tert-butyl ether, P 2 O 5 It was dried under vacuum at 40°C to obtain 43.1 g of white solid with a yield of 90.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com