Donor-acceptor compound light-emitting material based on dicyanopyrazine and dicyanobenzopyrazine derivative, and electroluminescent device for preparation
A technology of electroluminescent devices and pyrazine derivatives, applied in the field of organic electroluminescence, which can solve the problems of large efficiency roll-off and limited applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
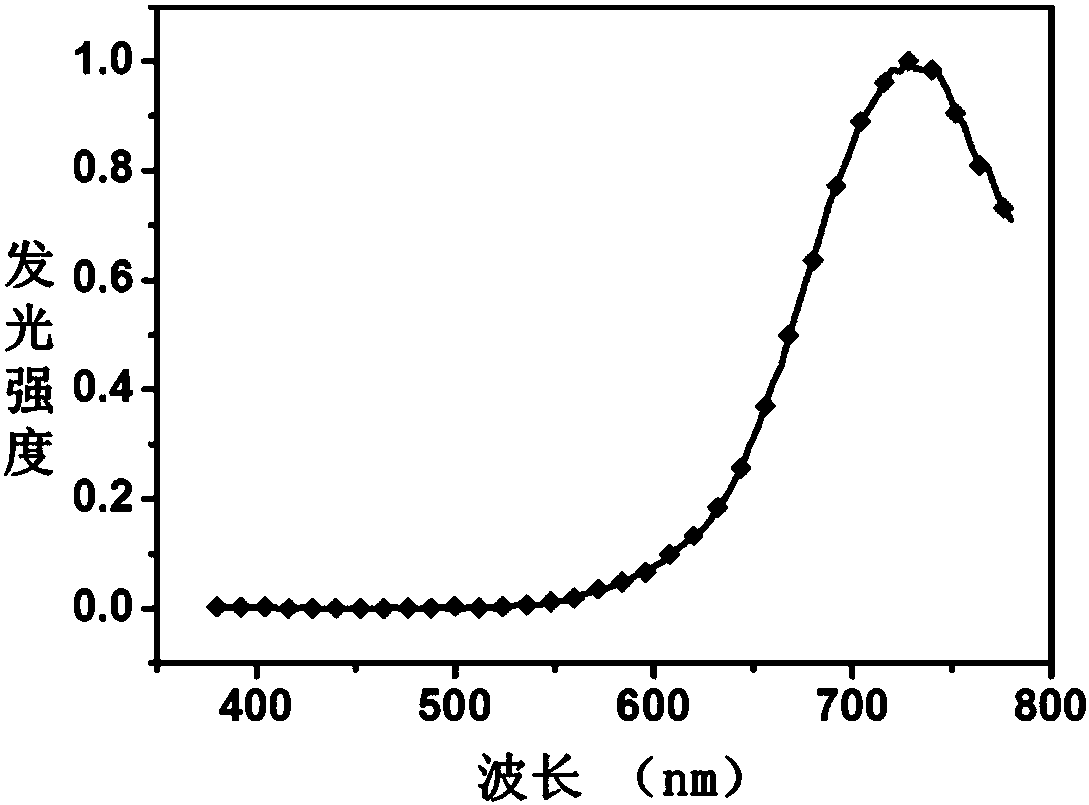
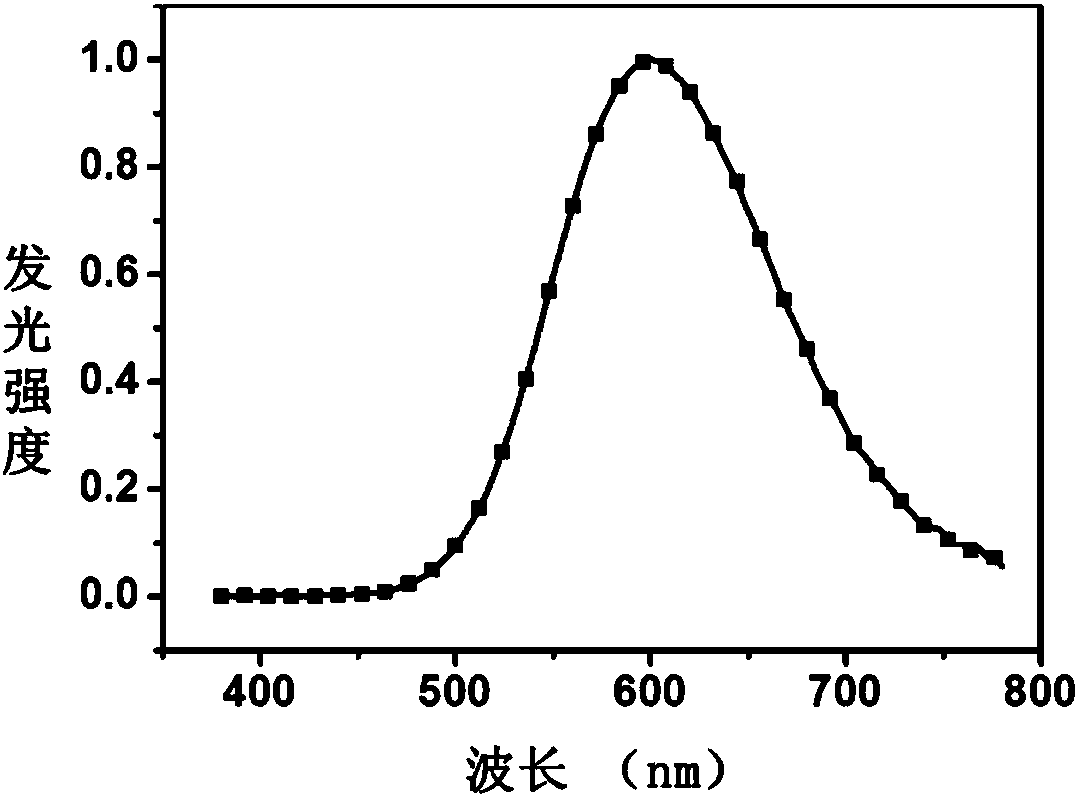
Image
Examples
Embodiment 1
[0032] Embodiment 1: the synthesis of compound 1:
[0033]
[0034] 4-bromophenacyl bromide (10mmol), diaminomaleonitrile (12mmol) was added in 50mL water, N 2 Under protection, cetyltrimethylammonium bromide (CTAB, 2.5mmol) was added, and the oil bath was heated to reflux for 8 hours to stop the reaction. After washing and drying, a yellow powdery brominated intermediate was obtained. Since this intermediate had extremely poor solubility, it was directly carried to the next step without further purification. Bromo intermediate (3mmol), carbazole (6.6mmol), cesium carbonate (24mmol), tris(dibenzylideneacetone) dipalladium (0.15mmol), tri-tert-butylphosphine (0.15mmol), o-xylene (80mL) into the three-necked bottle, N 2 Under protection, heat and reflux in an oil bath for 24 hours to stop the reaction, pour the reaction mixture into distilled water, extract with dichloromethane, concentrate and separate by column chromatography (silica gel, dichloromethane) and vacuum dry t...
Embodiment 2
[0035] Embodiment 2: the synthesis of compound 2:
[0036]
[0037] According to the synthesis of compound 1, the steps are the same, and compound 3,6-dimethylcarbazole is used to replace compound carbazole (the molar amount is the same) to obtain orange powder compound 2 (0.66g, yield 55%). Molecular ion mass: 399.19 (calculated value: 399.15); theoretical element content (%) C 26 h 17 N 5 : C, 78.18; H, 4.29; N, 17.53; Measured element content (%): C, 78.22; H, 4.35; N, 17.43. The above analysis results indicated that the obtained product was the expected product.
Embodiment 3
[0038] Embodiment 3: the synthesis of compound 3:
[0039]
[0040] According to the synthesis of compound 1, the steps are the same, and compound 2,7-dimethylcarbazole is used instead of compound carbazole to obtain orange powder compound 3 (0.63g, yield 53%), and the molecular ion mass determined by mass spectrometry is: 399.19 (calculated value: 399.15); theoretical element content (%) C 26 h 17 N 5 : C, 78.18; H, 4.29; N, 17.53; Measured element content (%): C, 78.25; H, 4.37; N, 17.38. The above analysis results indicated that the obtained product was the expected product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com