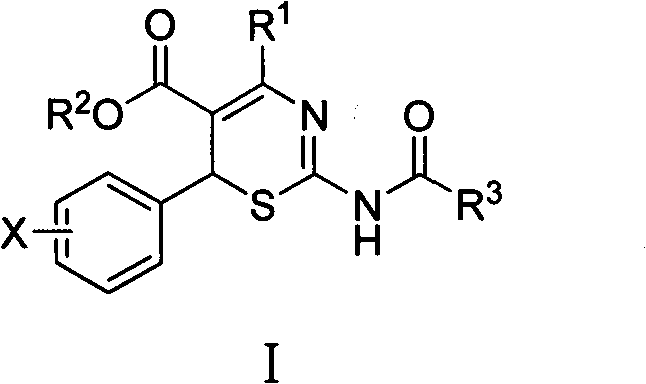
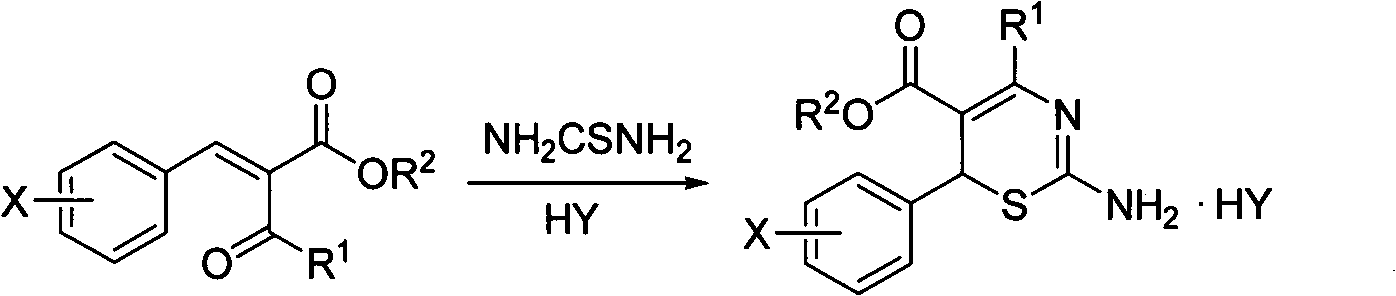
4-alkyl-6-aryl-2-acylamino-1,3-thiazine-5-formic ether, and preparation method and application thereof
A 2-OCH3, alkyl technology, applied in the field of 4-alkyl-6-aryl-2-amido-1,3-thiazine-5-carboxylate and its preparation and application, to achieve high resistance Effect of neuraminidase activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Preparation of ethyl 4-methyl-6-(4-methylphenyl)-2-acetylamino-1,3-thiazine-5-carboxylate
[0021]
[0022] (1) Preparation of ethyl 4-methyl-6-(4-methylphenyl)-2-amino-1,3-thiazine-5-carboxylate
[0023] Dissolve 0.01mol 2-(4-methylbenzylidene)ethyl acetoacetate and 0.012mol thiourea in 30mL methanol, 1mL concentrated hydrochloric acid, reflux, after the reaction is complete, part of the solvent is evaporated, and the solid is precipitated, recrystallized from ethanol, and dried Obtain 4-methyl-6-(4-methylphenyl)-2-amino-1,3-thiazine-5-carboxylic acid ethyl ester hydrochloride; dissolve the hydrochloride in ethanol, adjust pH with dilute NaOH solution 7-8, precipitated solid, recrystallized from ethanol, dried to obtain 4-methyl-6-(4-methylphenyl)-2-amino-1,3-thiazine-5-carboxylic acid ethyl ester, yield 74.5% , m.p.142~145℃. 1 H NMR (400M Hz, CDCl 3 ), δ: 1.24 (t, J=6.8Hz, 3H, CH 3 ), 2.14(s, 3H, COCH 3 ), 2.30(s, 3H, benzene ring CH 3 ), 2.55(s, 3H...
Embodiment 2
[0026] Example 2 Preparation of ethyl 4-methyl-6-(4-chlorophenyl)-2-acetylamino-1,3-thiazine-5-carboxylate
[0027]
[0028] (1) Preparation of ethyl 4-methyl-6-(4-chlorophenyl)-2-amino-1,3-thiazine-5-carboxylate
[0029] 0.004mol 2-(4-chlorobenzylidene)ethyl acetoacetate, 0.005mol thiourea and 30mL ethanol, 1mL hydrochloric acid, stirred and refluxed, after the reaction was completed, part of the solvent was evaporated, and the solid was precipitated, recrystallized from ethanol, and dried to obtain 4- Methyl-6-(4-chlorophenyl)-2-amino-1,3-thiazine-5-carboxylic acid ethyl ester hydrochloride; hydrochloride is dissolved in ethanol, dilute sodium carbonate solution to adjust pH 7~8 , a yellow solid was precipitated, recrystallized from ethanol, and dried to obtain ethyl 4-methyl-6-(4-chlorophenyl)-2-amino-1,3-thiazine-5-carboxylate with a yield of 87.7%, m.p.149 ~151°C. 1 H NMR (400M Hz, CDCl 3 ), δ: 1.23 (t, J=7.2Hz, 3H, CH 3 ), 2.48(s, 3H, CH 3 ), 4.15 (q, J=7.2Hz, 2H...
Embodiment 3
[0032] Example 3 Preparation of ethyl 4-methyl-6-(4-fluorophenyl)-2-acetylamino-1,3-thiazine-5-carboxylate
[0033]
[0034] (1) Preparation of ethyl 4-methyl-6-(4-fluorophenyl)-2-amino-1,3-thiazine-5-carboxylate
[0035] 0.005mol 2-(4-fluorobenzylidene)ethyl acetoacetate and 0.006mol thiourea, 30mL methanol, 1mL concentrated hydrochloric acid, stirred and refluxed, after the reaction was completed, part of the solvent was evaporated, and the solid was precipitated, recrystallized from ethanol, and dried to obtain 4 -Methyl-6-(4-fluorophenyl)-2-amino-1,3-thiazine-5-carboxylic acid ethyl ester hydrochloride; hydrochloride dissolved in ethanol, dilute sodium bicarbonate solution to adjust pH 7 ~8, a yellow solid was precipitated, recrystallized from ethanol, and dried to obtain pale yellow ethyl 4-methyl-6-(4-fluorophenyl)-2-amino-1,3-thiazine-5-carboxylate. Yield 85.7%, m.p.121-122°C. 1 H NMR (400M Hz, CDCl 3 ), δ: 1.23 (t, J=6.8Hz, 3H, CH 3 ), 2.49 (s, 3H, CH 3 ), 4.15...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com