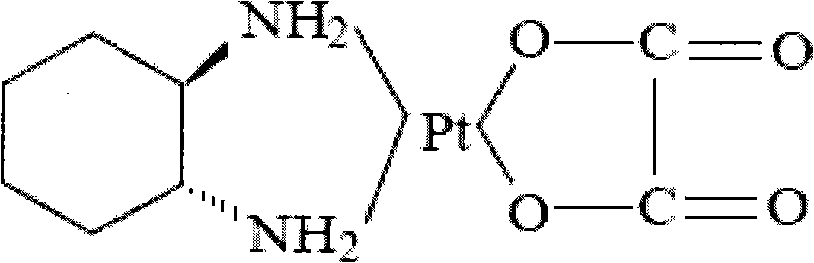
Method for preparing oxaliplatin
A technology of oxaliplatin and cis, which is applied in the field of preparation of oxaliplatin, can solve the problems of high chiral enantiomer residues and unfavorable production of chemical raw materials, and achieve high purity, increased yield, and low silver residues Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 20 g of cis-dichloro(L-trans-1,2-cyclohexanediamine) platinum was heated and stirred in purified water to dissolve, and the temperature was controlled at 60°C to 80°C. Slowly add 22 grams of silver nitrate under the condition of avoiding light and stirring, and continue to stir and react for 2 hours. After the reaction, the reaction solution was cooled to room temperature, filtered with suction, and the filtrate was collected. Add excess saturated sodium chloride solution to the filtrate, stir, filter, and collect the filtrate. Add ammonia water to the filtrate to adjust the pH=11, then add 14 grams of sodium oxalate, control the temperature at 65°C to 70°C, stir and react for 4 hours, concentrate the reaction solution under reduced pressure to 1 / 3 of the original volume, and cool it at 2 to 8°C Crystallize for 10 to 20 hours; when the crystallization is completed, the feed liquid is suction-filtered to obtain a filter cake, which is vacuum-dried at 75° C. to 80° C. fo...
Embodiment 2
[0046] 20 g of cis-dichloro(L-trans-1,2-cyclohexanediamine) platinum was heated and stirred in purified water to dissolve, and the temperature was controlled at 65°C to 70°C. Slowly add 20 grams of silver nitrate under the condition of avoiding light and stirring, and continue to stir and react for 2 hours. After the reaction, the reaction solution was cooled to room temperature, filtered with suction, and the filtrate was collected. Add excess saturated sodium chloride solution to the filtrate, stir, filter, and collect the filtrate. Add ammonia water to the filtrate to adjust pH=10, then add 10 grams of sodium oxalate, control the temperature at 60°C-80°C, stir and react for 4 hours, concentrate the reaction solution under reduced pressure to 1 / 3 of the original volume, and cool it at 2-8°C Crystallize for 10-20 hours; when the crystallization is completed, the feed liquid is suction-filtered to obtain a filter cake, which is vacuum-dried at 75° C. to 80° C. for 5 hours to ...
Embodiment 3
[0048] 20 g of cis-dichloro(L-trans-1,2-cyclohexanediamine) platinum was heated and stirred in purified water to dissolve, and the temperature was controlled at 60°C to 80°C. Slowly add 18 grams of silver nitrate under the condition of avoiding light and stirring, and continue to stir and react for 2 hours. After the reaction, the reaction solution was cooled to room temperature, filtered with suction, and the filtrate was collected. Add excess saturated sodium chloride solution to the filtrate, stir, filter, and collect the filtrate. Add ammonia water to the filtrate to adjust the pH=9, then add 7 grams of sodium oxalate, control the temperature at 60°C-80°C, stir and react for 4 hours, concentrate the reaction solution under reduced pressure to 1 / 3 of the original volume, and cool it at 2-8°C Crystallize for 10-20 hours; when the crystallization is completed, the feed liquid is suction-filtered to obtain a filter cake, which is vacuum-dried at 75° C. to 80° C. for 5 hours t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com