Anti-anthrax lethal factor chimeric monoclonal antibody, and preparation method and application thereof
A monoclonal antibody, anti-anthrax technology, applied in botany equipment and methods, biochemical equipment and methods, applications, etc., can solve the problems of human injury and limit the clinical application of LF8, and achieve high affinity and good safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
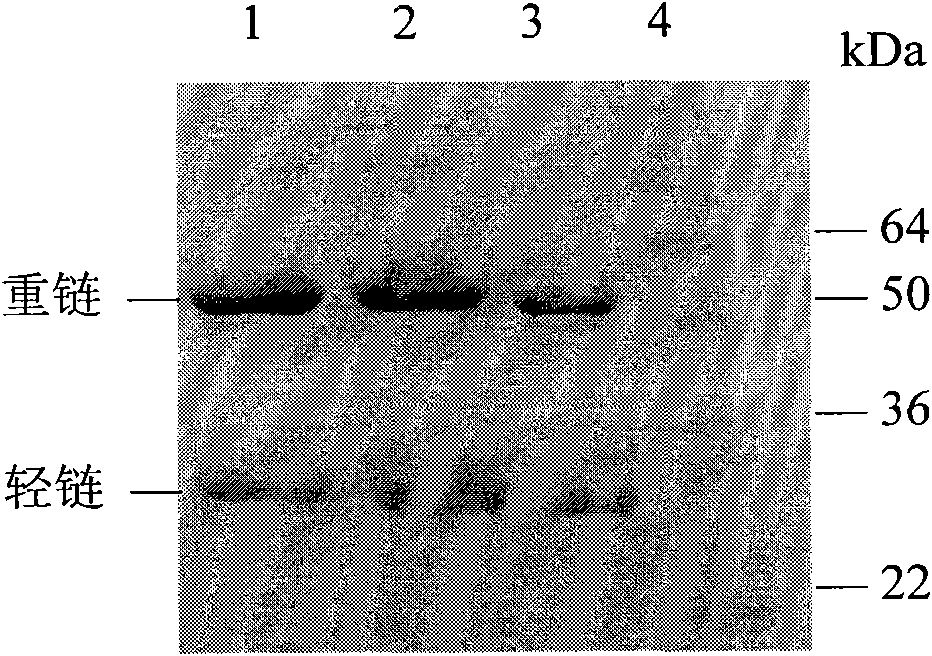
Image
Examples
Embodiment 1
[0043] Example 1 Preparation of HcLF8 Antibody
[0044] 1.1 Amplification of antibody variable region genes
[0045] method:
[0046] Hybridoma cells (LF8 cells) expressing LF8 antibody were cultured, total RNA was extracted with TRIzol reagent (Invitrogen, USA), and the OD was determined 260 and OD 280 . Using RNA as a template, reverse transcription was performed using SuperScript II reverse transcriptase (Invitrogen, USA) to obtain cDNA. Using cDNA as a template, specific primers were used to amplify the heavy chain variable region (variable region of heavy chain, V H ) and light chain variable region (variable region of light chain, V L ), the response looks like this:
[0047] (a) Primers:
[0048] V H Upstream: 5'-GGGGATATCCACCATGGRATGSAGCTGKGTMATSCTCTT-3'
[0049] Downstream: 5'-GACHGATGGGGSTGTYGTGCTAGCTGMRGAGACDGTGA-3'
[0050] V L Upstream: 5'-GGGGATATCCACCATGGATTTTCAAGTGCAGATTTTCAG-3'
[0051] Downstream: 5'-GGATACAGTTGGTGCAGTCGACTTACGTTTKATTTCCARCTT-3' ...
Embodiment 2
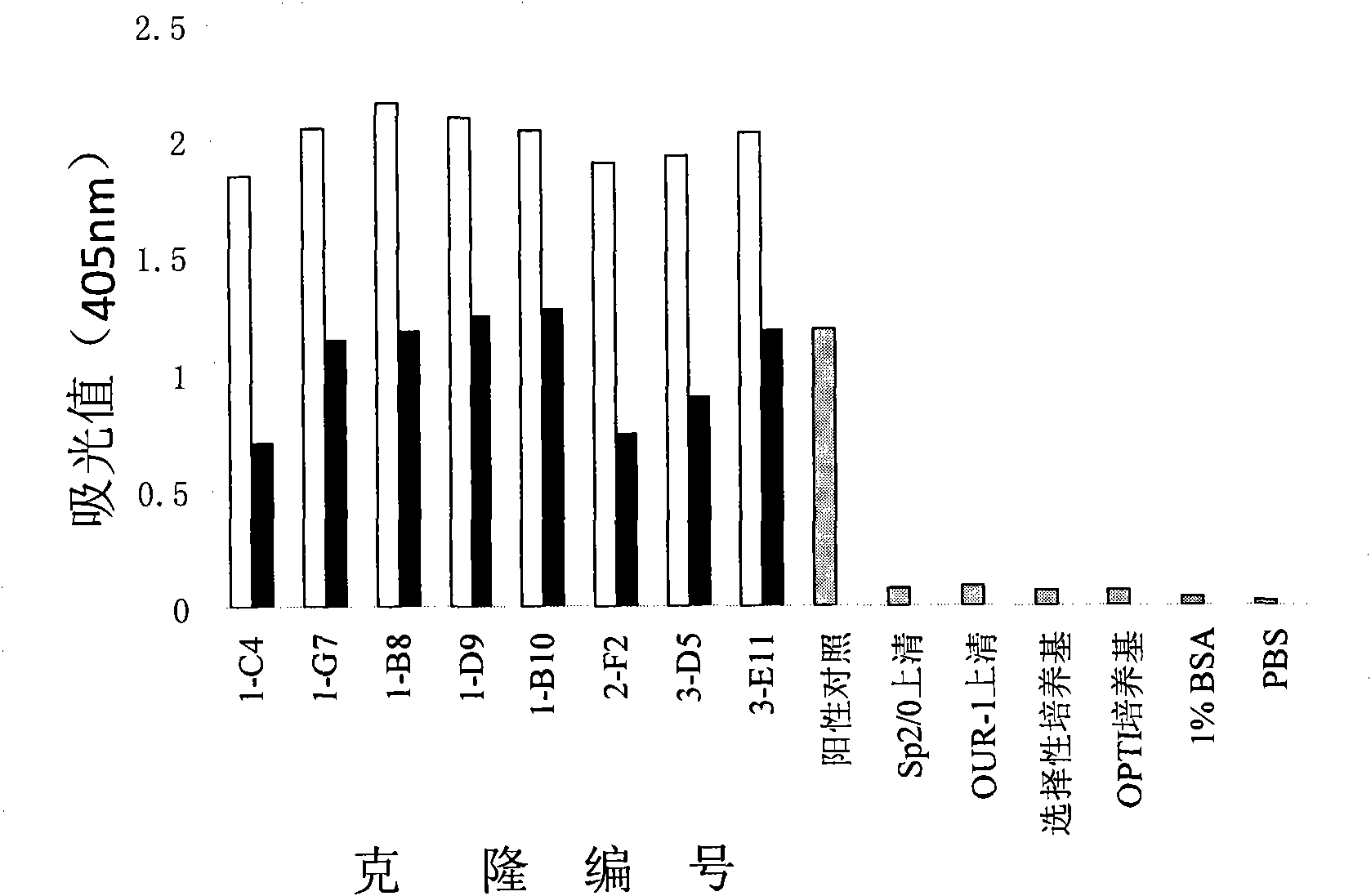
[0135] Example 2 HcLF8 Antibody Affinity Determination
[0136] method:
[0137] The affinity of HcLF8 antibody was determined by non-competitive immunosorbent assay (non-competitive ELISA).
[0138] 1) Coat a 96-well plate with two concentrations of recombinant LF protein (4 μg / ml and 2 μg / ml), overnight at 4°C;
[0139] 2) The next day, wash the plate twice with PBS solution (PBST) containing 0.1% Tween-20;
[0140] 3) Add 200 μl of 1% BSA solution to each well, and block for 1 hour at room temperature;
[0141] 4) Wash the plate twice with 0.1% PBST;
[0142] 5) Add HcLF8 antibody in gradient dilution, 50 μl per well, let stand at room temperature for 2 hours, and make 4 duplicate wells for each concentration; untransfected Sp2 / 0 cell supernatant, selective medium, 1% BSA, PBS are negative controls;
[0143] 6) Wash the plate 4 times with 0.1% PBST;
[0144] 7) Add alkaline phosphatase-labeled goat anti-human IgG (Fc specific), dilute 1:2000, 50 μl per well, and let st...
Embodiment 3
[0155] Example 3 HcLF8 Antibody Neutralization Test of Anthrax Lethal Toxin in Vitro—Neutralizing Effect of Different Concentration Antibodies on Lethal Toxin
[0156] 1. Methods and steps
[0157] (1) Take out J774A.1 cells from liquid nitrogen, and culture them in DMEM medium containing 10% FBS.
[0158] (2) When J774A.1 cells (ATCC) are in the logarithmic growth phase, adjust the concentration to 5×10 5 pieces / ml. The cells were transferred to a 96-well cell culture plate, 100 μl per well (5×10 4 cells).
[0159] (3) 12 hours after plating, 100 μl of HcLF8 antibody and lethal toxin mixture of different concentrations were added to each well. The lethal toxin concentration was fixed, and the final concentration of PA was set to 0.5 μg / ml and the final concentration of LF was set to 0.1 μg / ml according to the literature. According to the pre-experimental results, the initial concentration of HcLF8 antibody was set at 66nM (10μg / ml), diluted with culture medium and then mix...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com