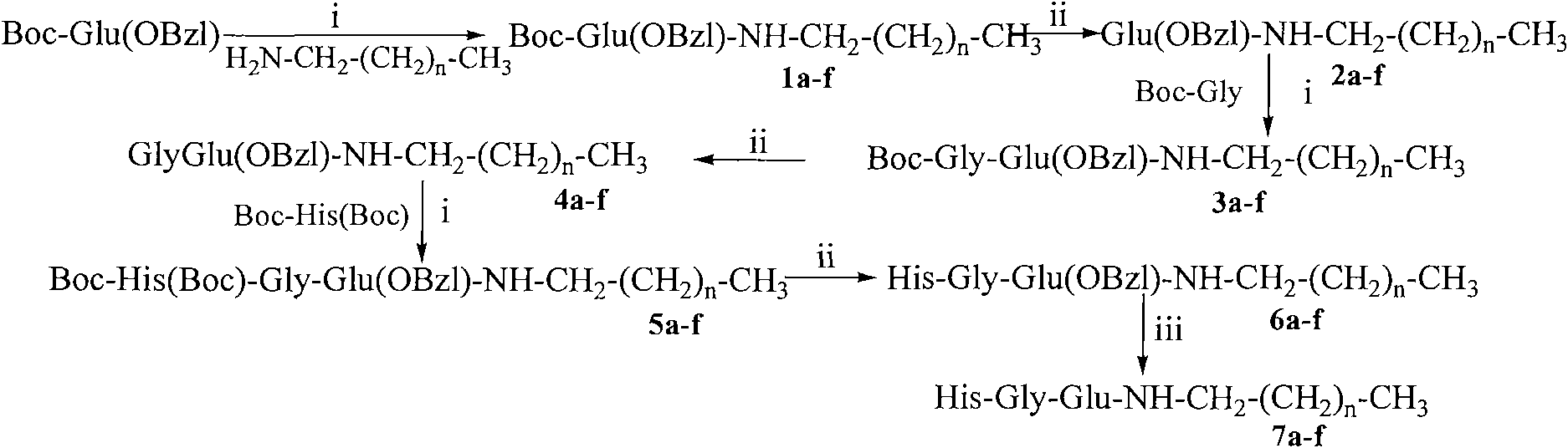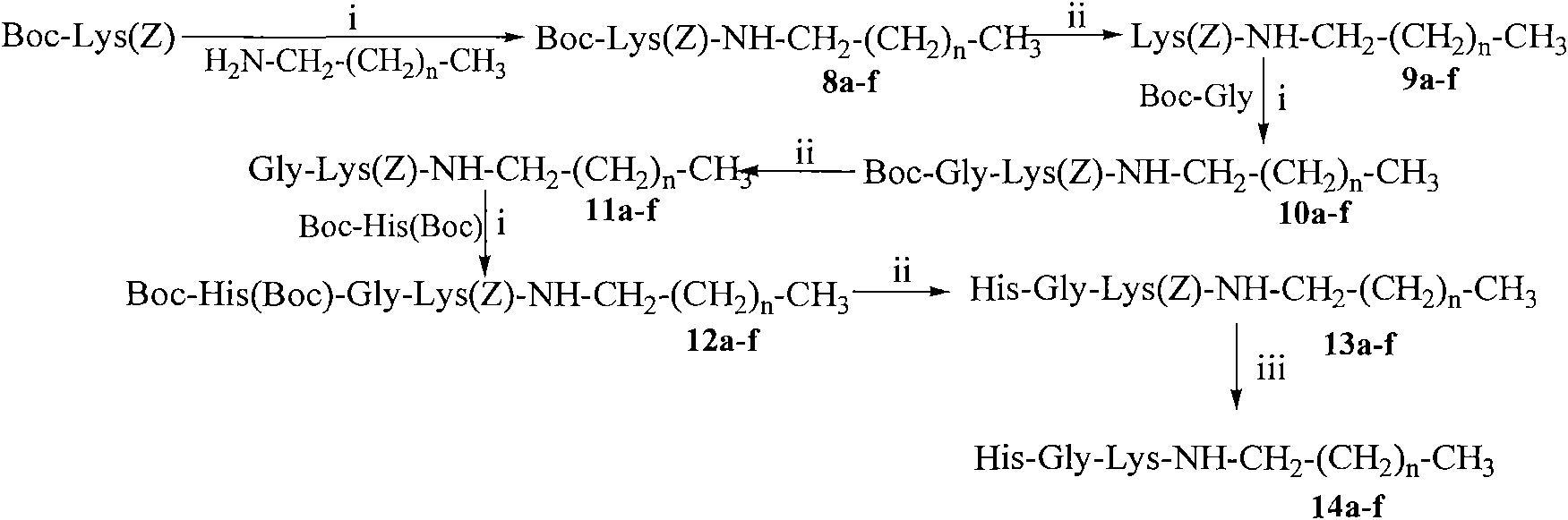Saturated fatty chain amine His-Gly-AA tripeptide amide, synthetic method and application thereof
A technology of his-gly-aa and aliphatic chain amine, which is applied in the field of biomedicine, can solve the problems of poor water solubility of cyclosporin A, unsatisfactory dosage form or curative effect, strong nephrotoxicity, etc., and achieve excellent immunosuppressive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
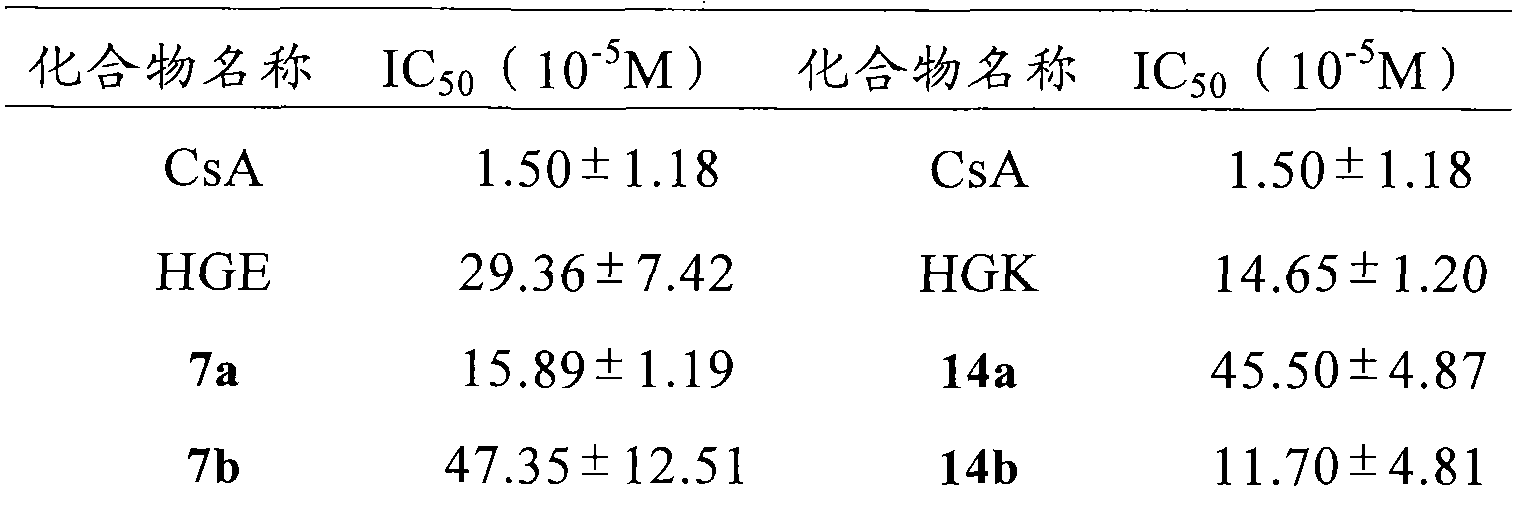
[0039] Example 1 Preparation of Boc-Glu(OBzl)-NHCH 2 (CH 2 ) 6 CH 3
[0040] 1.00g (2.96mmol) Boc-Glu (OBzl) was dissolved in 20ml of anhydrous THF, and 0.39g (2.96mmol) N-hydroxybenzotriazole (HOBt) was added to the resulting solution under ice cooling, and completely dissolve. After 10 minutes 0.74 g (3.55 mmol) of dicyclohexylcarbodiimide (DCC) were added. The reaction liquid (I) was obtained. Put 0.39g (2.96mmol) fatty amine CH under ice bath 3 (CH 2 ) 6 CH 2 NH 2 Suspend in 20ml of anhydrous THF, then add 1ml of N-methylmorpholine (NMM) to adjust the pH to 8-9. Stir for 35 minutes to obtain reaction solution (II). The reaction solution (I) was added to the reaction solution (II) under ice cooling, first stirred under ice cooling for 1 h, and then stirred at room temperature for 12 h, TLC (chloroform / methylamine, 10:1) showed that Boc-Glu (Z) disappeared. Dicyclohexylurea (DCU) was filtered off and THF was removed under reduced pressure. The residue was disso...
Embodiment 2
[0041] Example 2 Preparation of Boc-Glu(OBzl)-NHCH 2 (CH 2 ) 8 CH 3
[0042] According to the method for embodiment 1 by 1.00g (2.96mmol) Boc-Glu (OBzl) and 0.46g (2.96mmol) CH 3 (CH 2 ) 8 CH 2 NH 2 1.35 g (96%) of the title compound were obtained as a colorless solid. ESI-MS(m / z): 477[M+H] + , [α] 20 D =-4.1 (c=1.0, CH 3 OH).
Embodiment 3
[0043] Example 3 Preparation of Boc-Glu(OBzl)-NHCH 2 (CH 2 ) 10 CH 3
[0044] According to the method of embodiment 1 by 1.00g (2.96mmol) Boc-Glu (OBzl) and 0.55g (2.96mmol) CH 3 (CH 2 ) 10 CH 2 NH 2 1.45 g (97%) of the title compound were obtained as a colorless solid. ESI-MS(m / z): 505[M+H] + , [α] 20 D =-3.4 (c=1.0, CH 3 OH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com