Method for preparing manganese sulfate from waste byproducts obtained in the production of titanium white and low-grade pyrolusite
A technology of titanium dioxide and pyrolusite, which is applied to the removal of manganese sulfate and solid waste, can solve the problems of high energy consumption and long reaction time, and achieve the effect of low energy consumption and cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
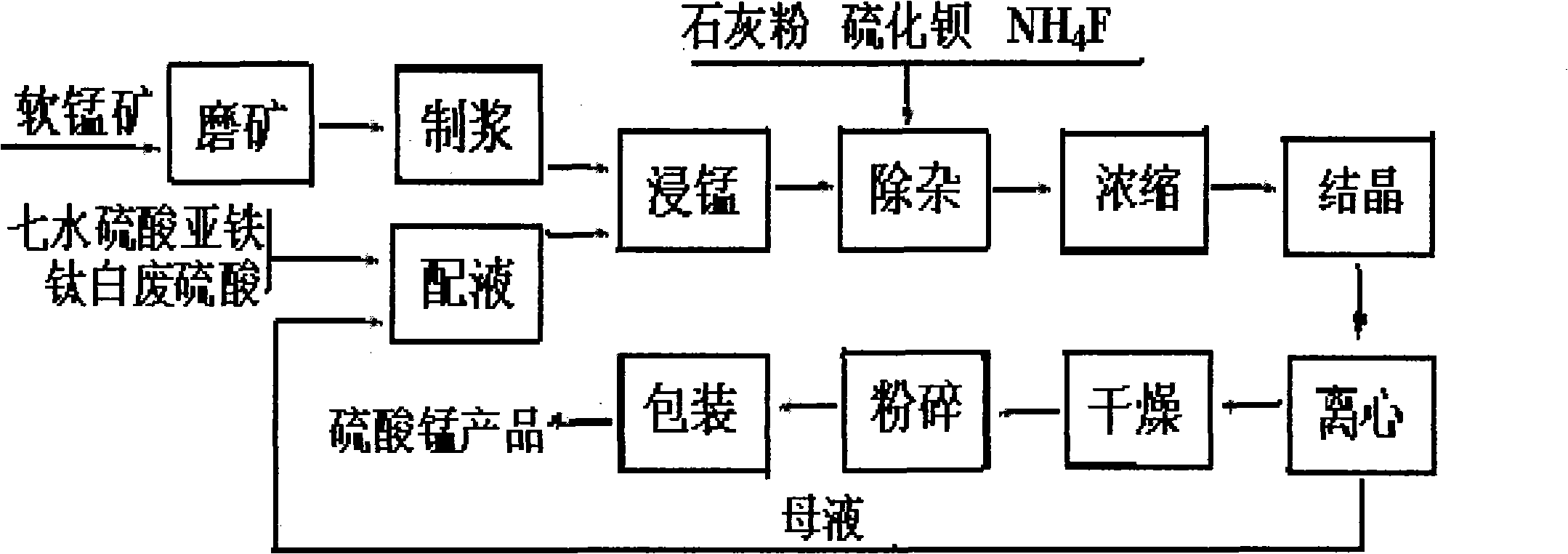
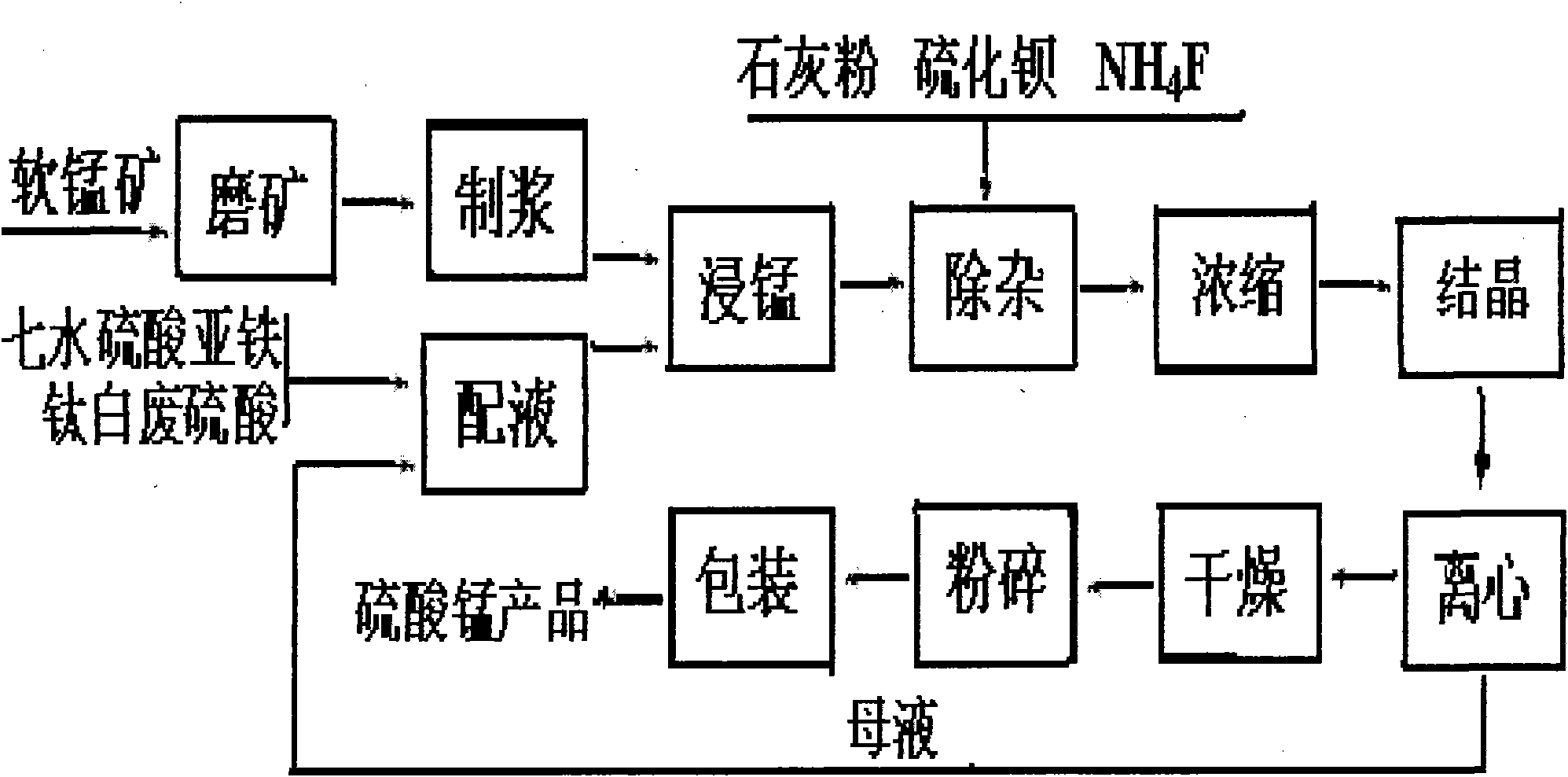
[0040] Grind low-grade pyrolusite with a manganese content of 19.3% to less than 100 meshes, weigh 10kg, and prepare manganese ore slurry according to the weight ratio of pyrolusite and titanium dioxide produced wastewater at 1:1.5; then weigh it according to the weight ratio. Get the waste sulfuric acid 19.56kg that the by-product ferrous sulfate heptahydrate of titanium dioxide production produces 19.5kg and titanium dioxide, add the waste water that titanium dioxide produces ferrous sulfate heptahydrate is dissolved, is mixed with ferrous sulfate mixed solution; Add manganese ore pulp and a quarter of ferrous sulfate mixed solution into the reaction kettle, stir for 35 minutes, then control the stirring speed to 200 rpm, and control the reaction temperature at 35°C, quickly mix the remaining manganese ore pulp and ferrous sulfate At the same time, the mixed solution is quickly sent to the reaction kettle to continue stirring and reacting for 3 hours; after the reaction is co...
Embodiment 2
[0042] Grind the low-grade pyrolusite with a manganese content of 18.7% to less than 100 meshes, weigh 10kg, prepare manganese ore slurry according to the weight ratio of pyrolusite and titanium dioxide produced wastewater at 1:1, and then weigh it according to the weight ratio. Get the waste sulfuric acid 18.67kg that the by-product ferrous sulfate heptahydrate produced by titanium dioxide produces 18.8kg and titanium dioxide, add the waste water that titanium dioxide produces ferrous sulfate heptahydrate is dissolved, be mixed with ferrous sulfate mixed solution; Add the manganese ore slurry and 1 / 4 of the ferrous sulfate mixed solution into the reaction kettle, stir for 30 minutes, then control the stirring speed to 100 rpm, and control the reaction temperature at 40°C, quickly mix the remaining manganese ore slurry and ferrous sulfate The mixed solution is quickly sent to the reaction kettle to continue stirring and reacting for 2 hours; after the reaction is completed, the...
Embodiment 3
[0044]Grind the low-grade pyrolusite with a manganese content of 18.35% to less than 100 meshes, weigh 10kg, prepare manganese ore slurry according to the weight ratio of pyrolusite and titanium dioxide wastewater production at 1:0.8, and then weigh it according to the weight ratio. Get the waste sulfuric acid 18.56kg that the by-product ferrous sulfate heptahydrate of titanium dioxide production produces 18.5kg and titanium dioxide, add titanium dioxide production waste water ferrous sulfate heptahydrate is dissolved, be mixed with ferrous sulfate mixed solution; Add the manganese ore slurry and a quarter of the ferrous sulfate mixed solution into the reaction kettle, stir for 25 minutes, then control the stirring speed to 70 rpm, control the reaction temperature at 26°C, and quickly put the remaining manganese ore slurry and ferrous sulfate solution At the same time, it is quickly sent to the reaction kettle to continue stirring and reacting for 4 hours; after the reaction is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com