1-(3-aminopropyl) piperazine-4-aminoamide compound as well as preparation method and application thereof
A compound, the technology of piperidinecarboxamide, which is applied to 1-(3-aminopropyl)piperazine-4-aminoamide compounds and the fields of preparation and application thereof, can solve drug resistance, high toxicity of antiviral drugs, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1, 1-acetyl-N-[3-(4-benzylpiperazin-1-yl)propyl]-N-(3-chloro-4-methylphenyl)piperidine-4-methyl Synthesis of amides (DMX-B01) (in formula I, X is methylene, R 1 for phenyl compounds)
[0049] 1. Preparation of N-(3-chloropropyl)-3-chloro-4-methylaniline
[0050] To a solution of 3-chloro-4-methylaniline (7.08 g, 50 mmol) in DMF (5 mL) was added 1-bromo-3-chloropropane (15.5 mL, 50 mmol), potassium iodide (0.83 g, 5 mmol) and triethyl Amine (30 mL). The mixture was stirred at room temperature for 3 days. Then the solvent was evaporated to dryness, diluted with diethyl ether (100 mL) and washed with brine, the separated organic phase was dried over sodium sulfate and concentrated under reduced pressure. The concentrate was separated by column chromatography (ethyl acetate / petroleum ether=1:25, v / v), and the light brown oil was obtained as N-(3-chloropropyl)-3-chloro-4-methylaniline (10 g, yield 92%).
[0051] The structural confirmation data are as follows: ...
Embodiment 2
[0072] Example 2, 1-acetyl-N-[3-(4-benzoylpiperazin-1-yl)propyl]-N-(3-chloro-4-methylphenyl)piperidine-4- Synthesis of formamide (DMX-B02) (in formula I, X is a carbonyl group, R1 is a phenyl compound)
[0073] According to the method for preparing the target compound DMX-B01 in Example 1, by 1-acetyl-N-(3-chloro-4-methylphenyl)-N-(3-chloropropyl)-4-piperidinecarboxamide (0.37g, 1.0mmol) was reacted with 1-benzoylpiperazine trifluoroacetate (0.45g, 1.5mmol) (provided by Amber Technology Co., Ltd.) to obtain 0.2g of yellow foamy solid, with a yield of 38%.
[0074] The structural confirmation data are as follows:
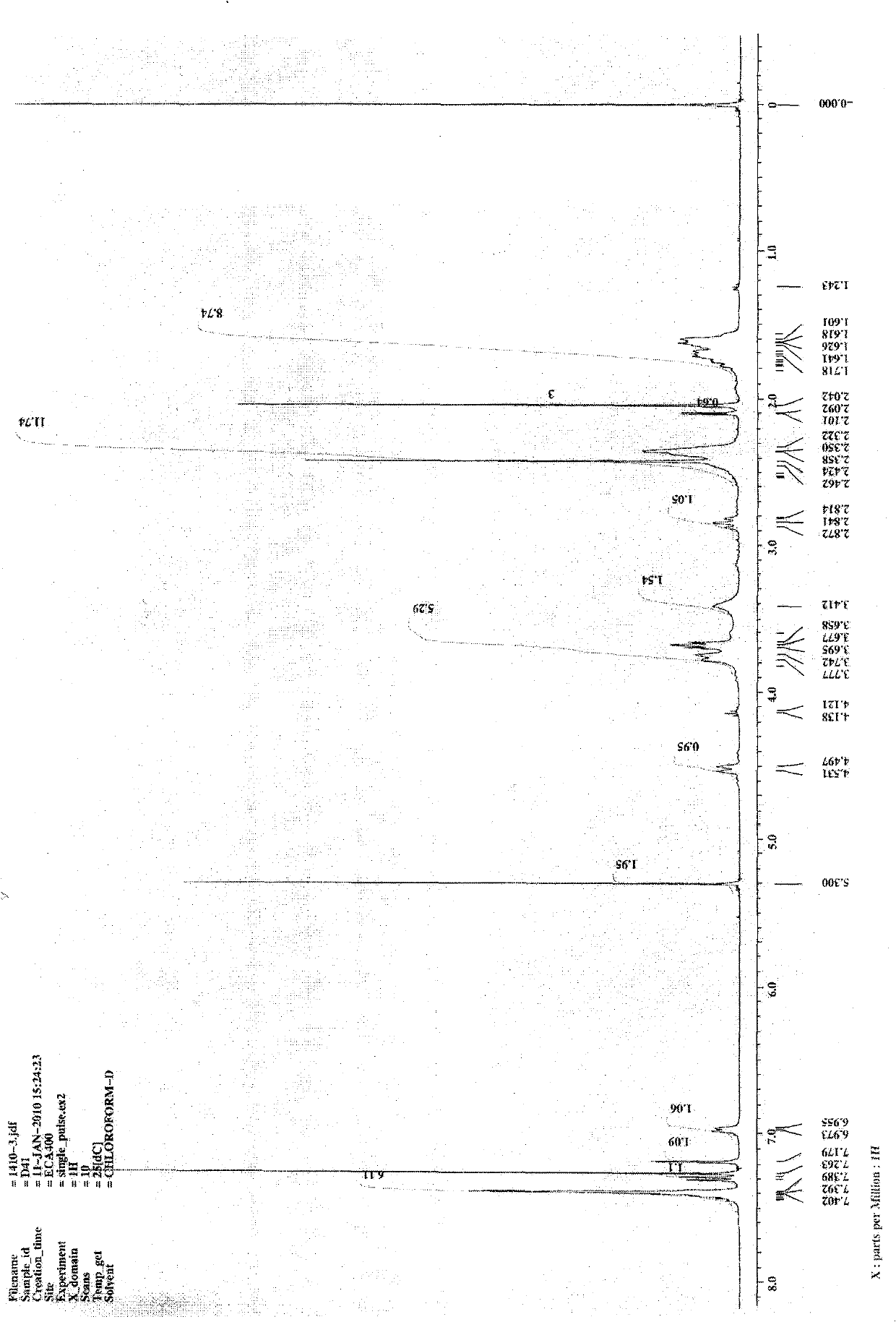
[0075] 1 H NMR (400Hz, CDCl 3 ): δ7.31(m, 6H), 7.17(m, 1H), 6.97(d, J=6.4Hz, 1H), 4.52(d, J=13.6Hz, 1H), 3.74(m, 3H), 3.51 (m, 2H), 2.47(m, 4H), 2.42(s, 3H), 2.34(m, 6H), 2.04(s, 3H), 1.70(m, 6H);
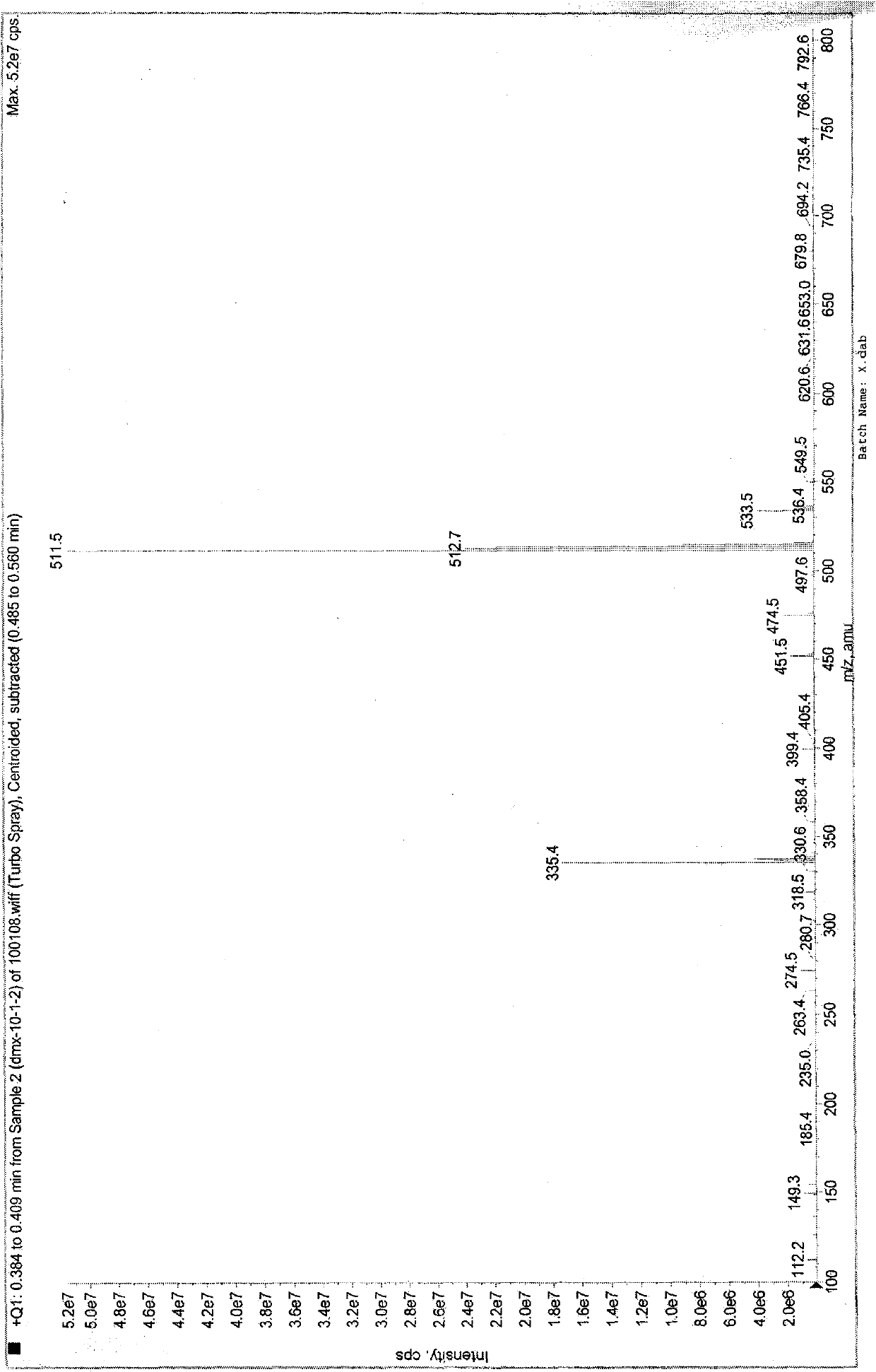
[0076] MS (Turbo Spray) m / z (M+1): 525.6.
Embodiment 3
[0077] Example 3, 1-acetyl-N-{3-[1-(2-chloro-thiazol-5-yl-methylene)piperazin-1-yl]propyl}-N-(3-chloro- Synthesis of 4-methylphenyl)piperidine-4-formamide (DMX-B03) (in formula I, X is methylene, R 1 is 5-2-chloro-thiazolyl compound)
[0078] According to the above-mentioned method for preparing the target compound DMXB01, from 1-acetyl-N-(3-chloro-4-methylphenyl)-N-(3-chloropropyl)-4-piperidinecarboxamide (0.37g, 1.0mmol) was reacted with 1-(2-chloro-thiazol-5-yl-methyl)piperazine dihydrochloride (0.26g, 0.9mmol) (provided by Amber Technology Co., Ltd.) to obtain 0.35g yellow foamy solid, Yield 70%.
[0079] The structural confirmation data are as follows:
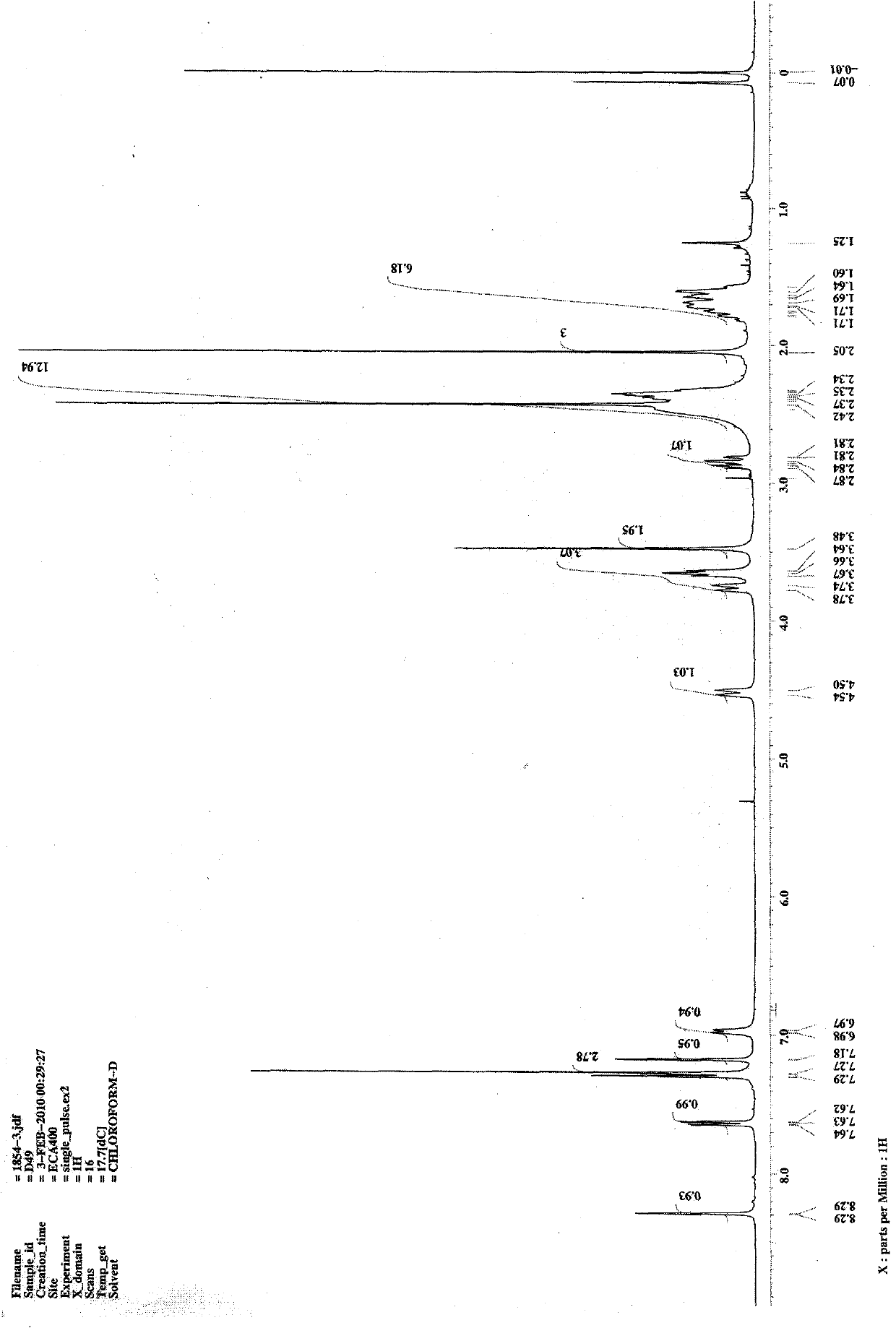
[0080] 1 H NMR (400Hz, CDCl 3 ): δ7.34(m, 2H), 7.18(s, 1H), 6.98(d, J=1.72Hz, 1H), 4.52(d, J=13.4Hz, 1H), 3.77(d, J=13.7Hz , 3H), 3.65(m, 4H), 2.85(m, 1H), 2.47(m, 3H), 2.42(s, 3H), 2.34(m, 6H), 2.05(s, 3H), 1.66(m, 6H);
[0081] MS (Turbo Spray) m / z (M+1): 554.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com