Benzisoselenazolone difluorocytidine compound as well as preparation method and application thereof
A technology of benzisoselazolone difluorocytidine and benzisoselazole, which is applied in the field of benzisoselazolone difluorocytidine compounds and their preparation, and can solve the problems of reducing toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1 Preparation of Compound 1-4
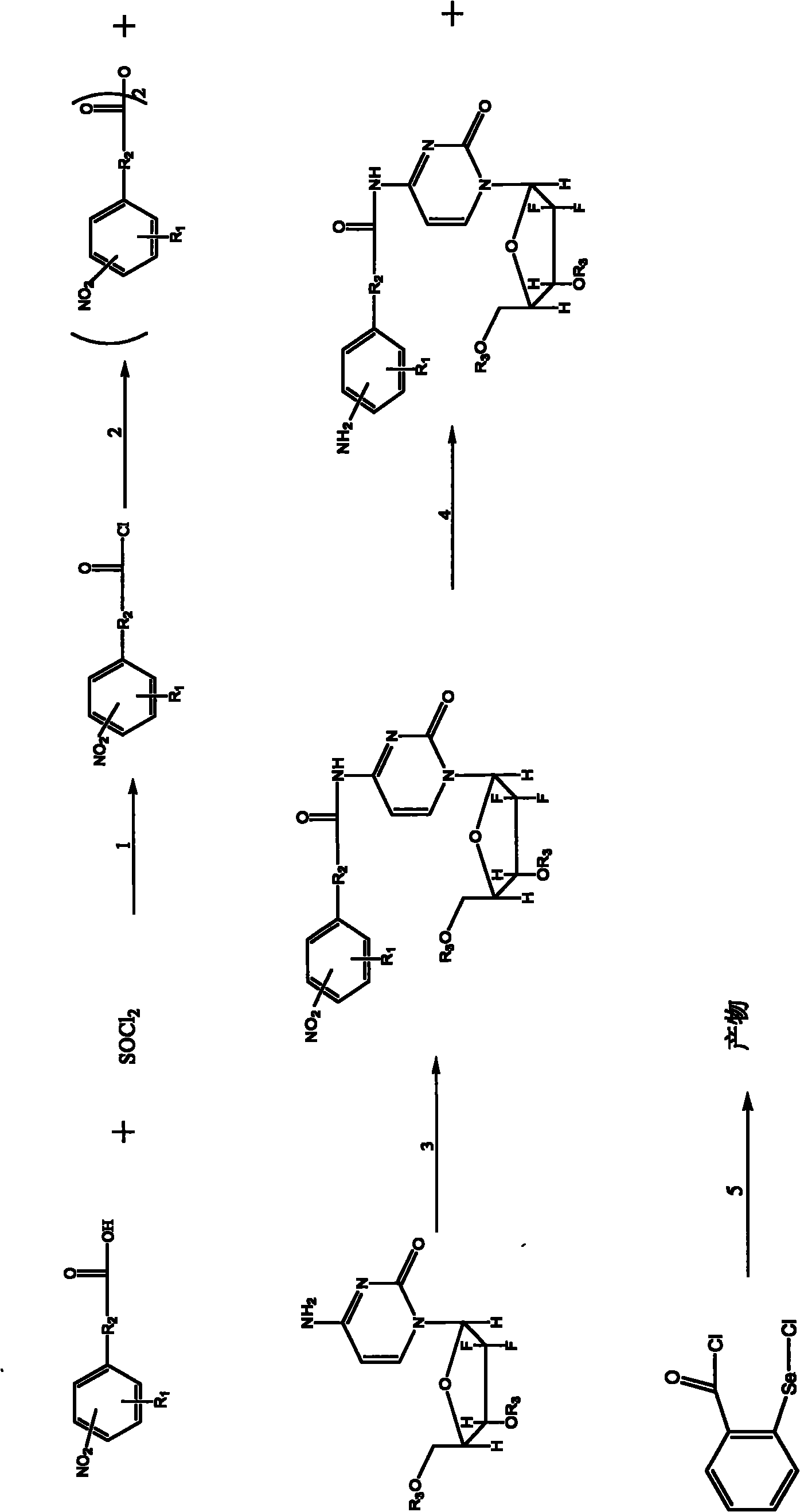
[0076] The synthetic route of compound 1-4 is as follows figure 2 Shown, wherein, R1 is selected from-H,-Cl,-F,-CH3, preparation process is:
[0077] 1) Take 4mmol 2-R14-nitrobenzoic acid (R1 is selected from -H, -Cl, -F, -CH3) and place it in 25ml of thionyl chloride to reflux for 3h, spin dry the excess thionyl chloride, and the remaining oily Liquid; add an appropriate amount of dichloromethane, dissolve, and spin dry to obtain a light yellow solid, that is, 2-R1-4-nitrophenylformyl chloride (R1 is selected from -H, -Cl, -F, -CH3), and It was dissolved in 20ml of dichloromethane and set aside;
[0078] 2) Weigh 0.132g (0.4mmol) tetrabutylamine bromide (TBAP) and dissolve it in 10ml of dichloromethane, and slowly add 5ml of 20% NaOH solution dropwise, cool to -10°C, stir; then slowly Add dropwise the 2-R1-4-nitrophenylformyl chloride dichloromethane solution prepared in step 1), keep the reaction system at -10°C, and conti...
Embodiment 2
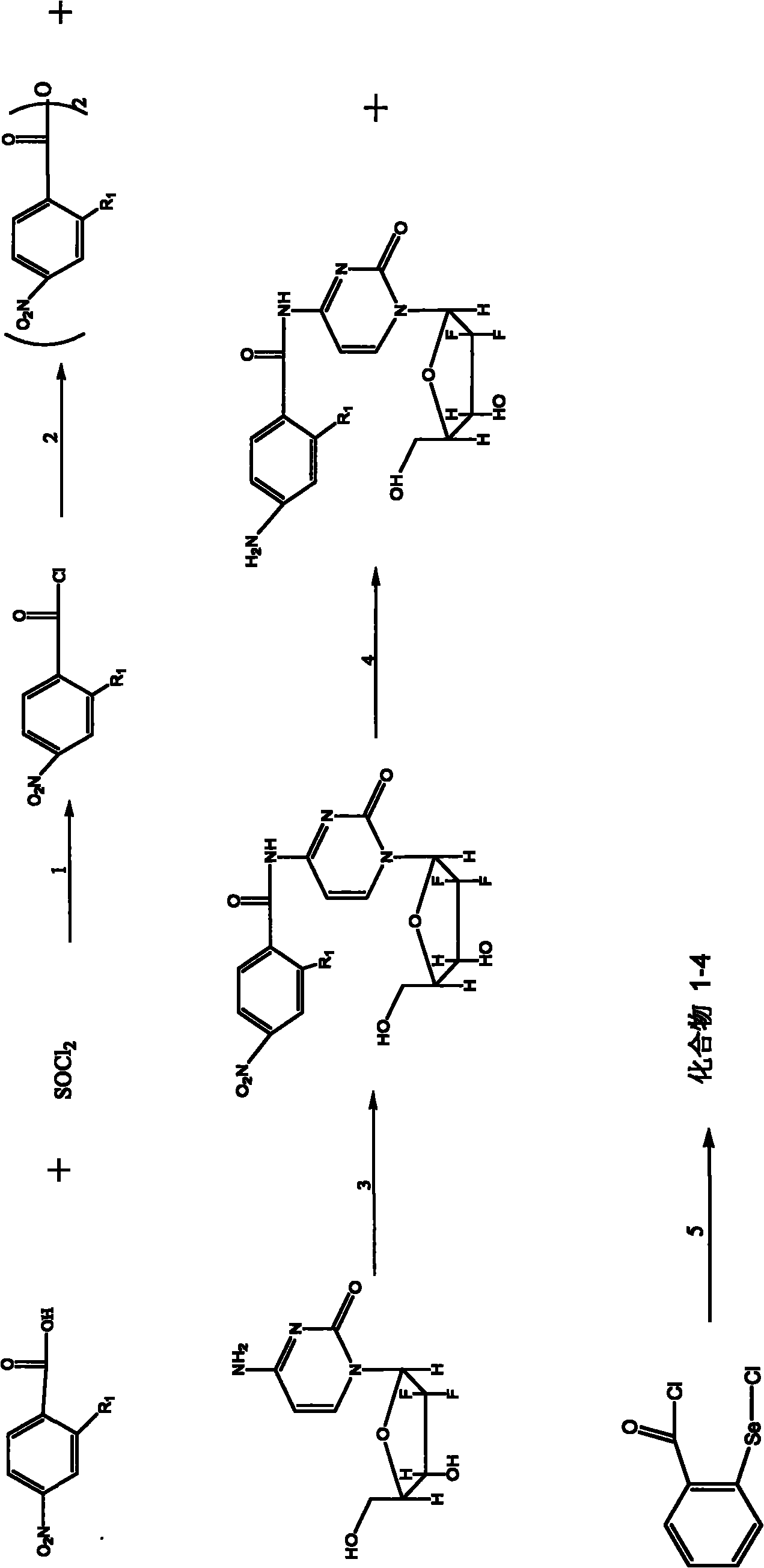
[0082] Example 2 Preparation of Compound 5-6
[0083] The synthetic route of compound 5-6 is as follows image 3 As shown, the preparation conditions of compound 5 are the same as in Example 1, and compound 5 is placed in a hydrogenator, and then a catalytic amount of Ni is added to react for 20 minutes to obtain compound 6.
Embodiment 3
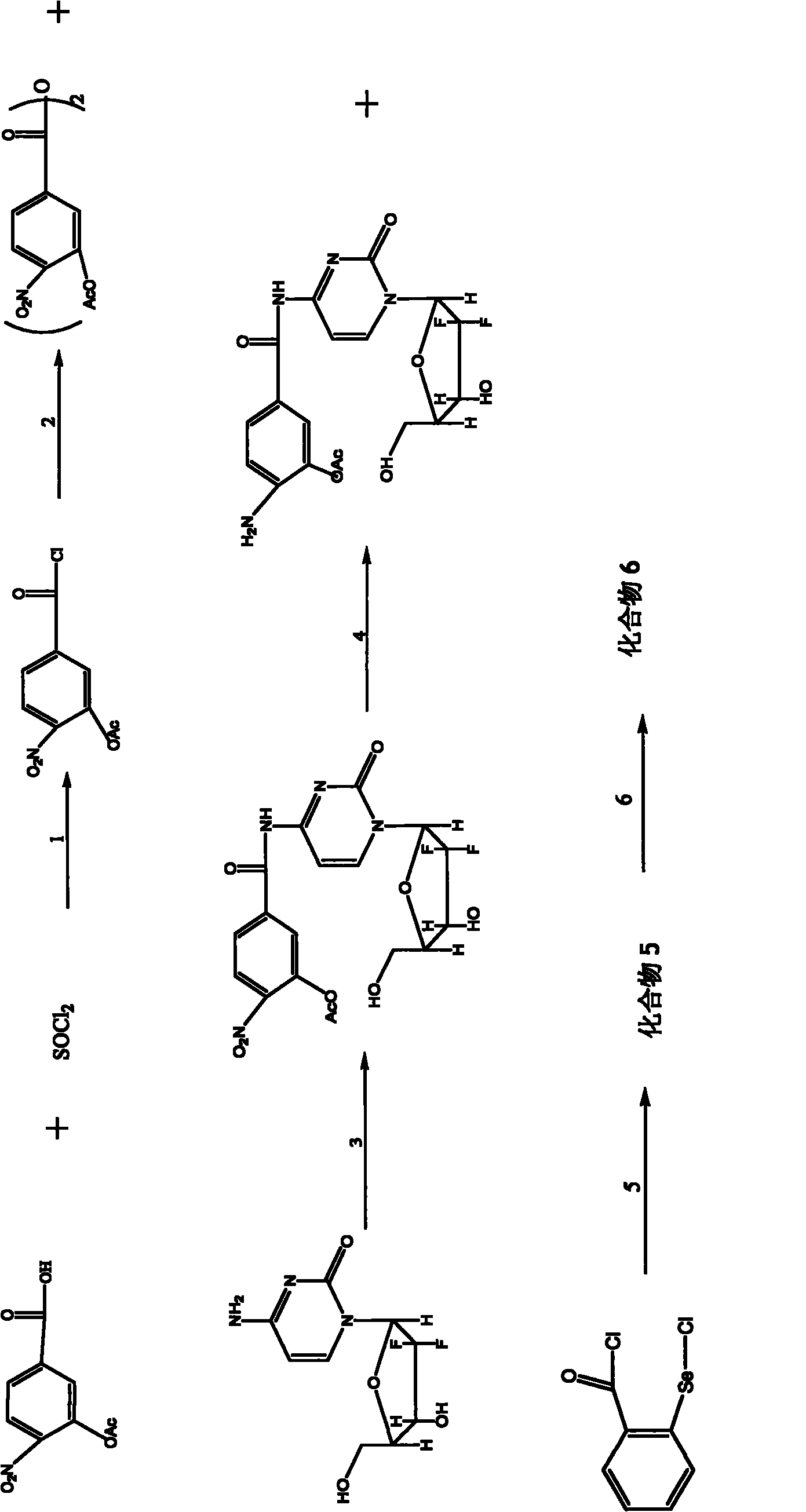
[0084] Example 3 Preparation of Compound 7-10
[0085] The synthetic route of compound 7-10 is as follows Figure 4 Shown, wherein, R1' is selected from -H, -Cl, -F, -CH3, 2-R1-5-nitrophenyl acid chloride (R1 is selected from -H, -Cl, -F, -CH3), 2 , 2'-diR1-5,5'-dinitrobenzoic anhydride, N-(5-nitro-2-R1phenylyl)-2'-deoxy-2',2'-difluorocytidine , N-(5-amino-2-R1 phenylacyl)-2'-deoxy-2', 2'-difluorocytidine and the preparation conditions of compound 7-10 are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com