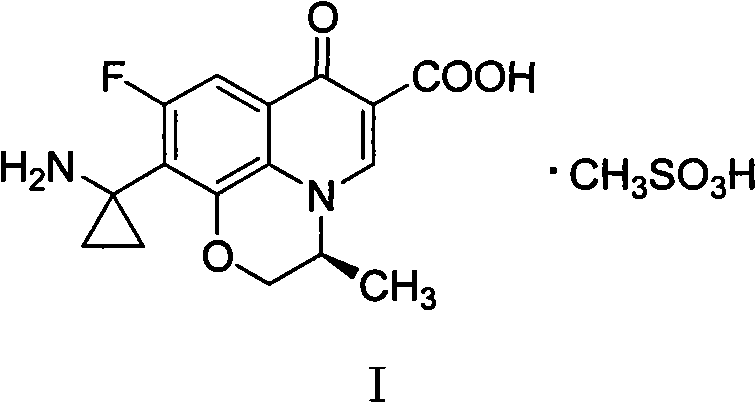
Method for purifying pazufloxacin mesylate
A technology of pazufloxacin mesilate and pazufloxacin, which is applied in the field of preparation of pazufloxacin mesilate, can solve the problem that the quality of pazufloxacin mesilate cannot reach that of pazufloxacin mesilate Quality standard requirements and other issues, to achieve the effect of high economic and practical value, long shelf life and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Add pazufloxacin mesylate (10 g) and N,N-dimethylacetamide (45 mL) into a 100 mL single-necked bottle, heat to an internal temperature of 120° C. under electromagnetic stirring, and dissolve completely. Naturally lowered to room temperature, cooled to 0°C, and left overnight. Filter, wash the filter cake with ethanol (3x15mL), and vacuum-dry at 60°C for 12 hours to obtain 7.5g of pazufloxacin mesylate (purification rate: 75%).
Embodiment 2
[0043] Add pazufloxacin mesylate (10g) and N,N-dimethylacetamide (20mL) into a 100mL single-necked bottle, heat to 60°C, dissolve; cool to 0°C, stand overnight; filter, wash with 5mL ethanol Washing, repeated washing for a total of 3 times, vacuum drying at 50°C for 10 hours to obtain the refined product of pazufloxacin mesylate.
Embodiment 3
[0045]Add 10g of pazufloxacin mesylate and 200ml of N,N-dimethylacetamide into a 500mL single-necked bottle, heat to 135°C, dissolve; cool to 10°C, leave overnight; filter, wash with 50mL of ethanol, repeat washing A total of 3 times, vacuum drying at 105°C for 24 hours to obtain the refined product of pazufloxacin mesylate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com