Amino acid composition containing new antioxidant
An antioxidant and composition technology, applied in the field of medicine, can solve problems such as huge investment, achieve the effects of saving transformation investment, solving harm to human body, and eliminating process loopholes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] 1. Prescription:
[0110] Isoleucine 9.10g Leucine 12.90g Lysine Acetate 10.00g
[0111] Methionine 4.40g Phenylalanine 7.00g Threonine 7.50g
[0112] Tryptophan 1.30g Valine 14.00g Alanine 7.10g
[0113] Arginine 9.00g Aspartic Acid 1.00g Glutamic Acid 0.50g
[0114] Histidine 5.00g Proline 5.00g Serine 1.70g
[0115] Tyrosine 0.40g Glycine 7.00g Cysteine 0.35g
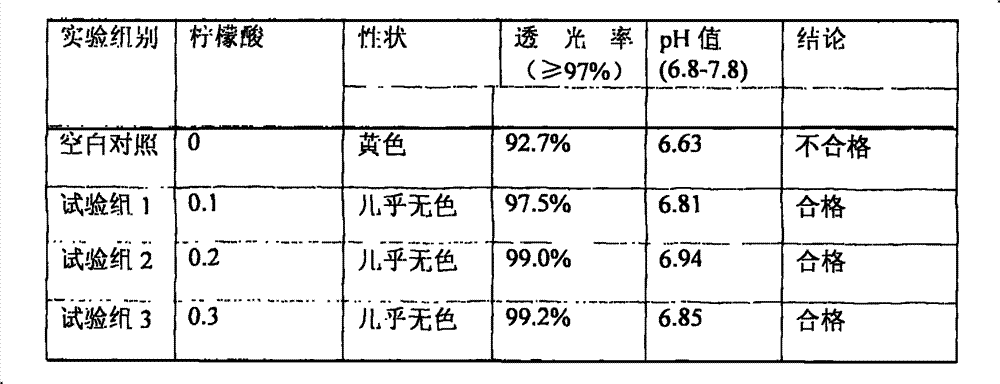
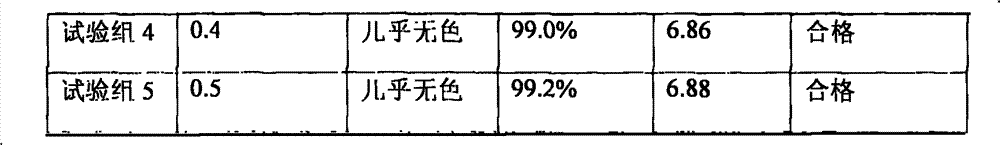
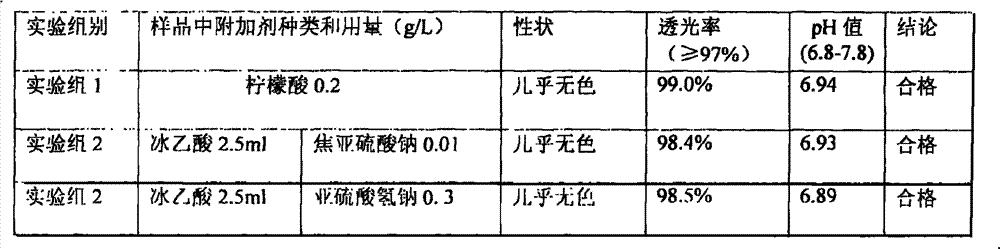
[0116] Citric acid 0.1g water for injection appropriate amount
[0117] Full volume 1000ml
[0118] 2. Preparation process
[0119] (1) Weigh each raw material and auxiliary material according to the prescription;
[0120] (2) Take about 80% of the total amount of water for injection, heat it to above 95°C, keep it warm for more than 30 minutes with nitrogen, control the water temperature at 80-85°C, add citric acid under the protection of nitrogen flow, and dissolve;
[0121] (3) Under the protection of nitrogen flow, add inhalantic acid, tyrosine, leucine, phenylalanine, glutamic acid, isoleucine, ...
Embodiment 2
[0126] 1. Prescription:
[0127] Isoleucine 8.19g Leucine 11.61g Lysine Acetate 9.00g
[0128] Methionine 3.96g Phenylalanine 6.30g Threonine 6.75g
[0129] Tryptophan 1.17g Valine 12.60g Alanine 6.39g
[0130] Arginine 8.10g Aspartic acid 0.90g Glutamic acid 0.45g
[0131] Histidine 4.50g Proline 4.50g Serine 1.53g
[0132] Tyrosine 0.36g Glycine 6.30g Cysteine 0.32g
[0133] Citric acid 0.2g water for injection appropriate amount
[0134] The total volume is 1000ml.
[0135] 2. Preparation process
[0136] (1) Weigh each raw material and auxiliary material according to the prescription;
[0137] (2) Take about 80% of the total amount of water for injection, heat it to above 95°C, keep it warm for more than 30 minutes with nitrogen, control the water temperature at 80-85°C, add citric acid under the protection of nitrogen flow, and dissolve;
[0138] (3) Under the protection of nitrogen flow, add inhalantic acid, tyrosine, leucine, phenylalanine, glutamic acid, isol...
Embodiment 3
[0143] 1. Prescription:
[0144] Isoleucine 91.0g Leucine 129.0g Lysine Acetate 100.0g
[0145] Methionine 44.0g Phenylalanine 70.0g Threonine 75.0g
[0146] Tryptophan 13.0g Valine 140.0g Alanine 71.0g
[0147] Arginine 90.0g Aspartic acid 10.0g Glutamic acid 5.0g
[0148] Histidine 50.0g Proline 50.0g Serine 17.0g
[0149] Tyrosine 4.0g Glycine 70.0g Cysteine 3.5g
[0150] Citric acid 2.0g water for injection appropriate amount
[0151] Full volume 10000ml
[0152] 2. Preparation process
[0153] (1) Weigh each raw material and auxiliary material according to the prescription;
[0154] (2) Add 80% water for injection in the preparation tank, heat it, fill it with nitrogen for 30 minutes, keep the temperature above 85°C, add citric acid under the protection of nitrogen filling, stir and dissolve;
[0155] (3) Under the protection of nitrogen flow, add inhalantic acid, tyrosine, leucine, phenylalanine, glutamic acid, isoleucine, valine, methionine, histidine, alanine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com