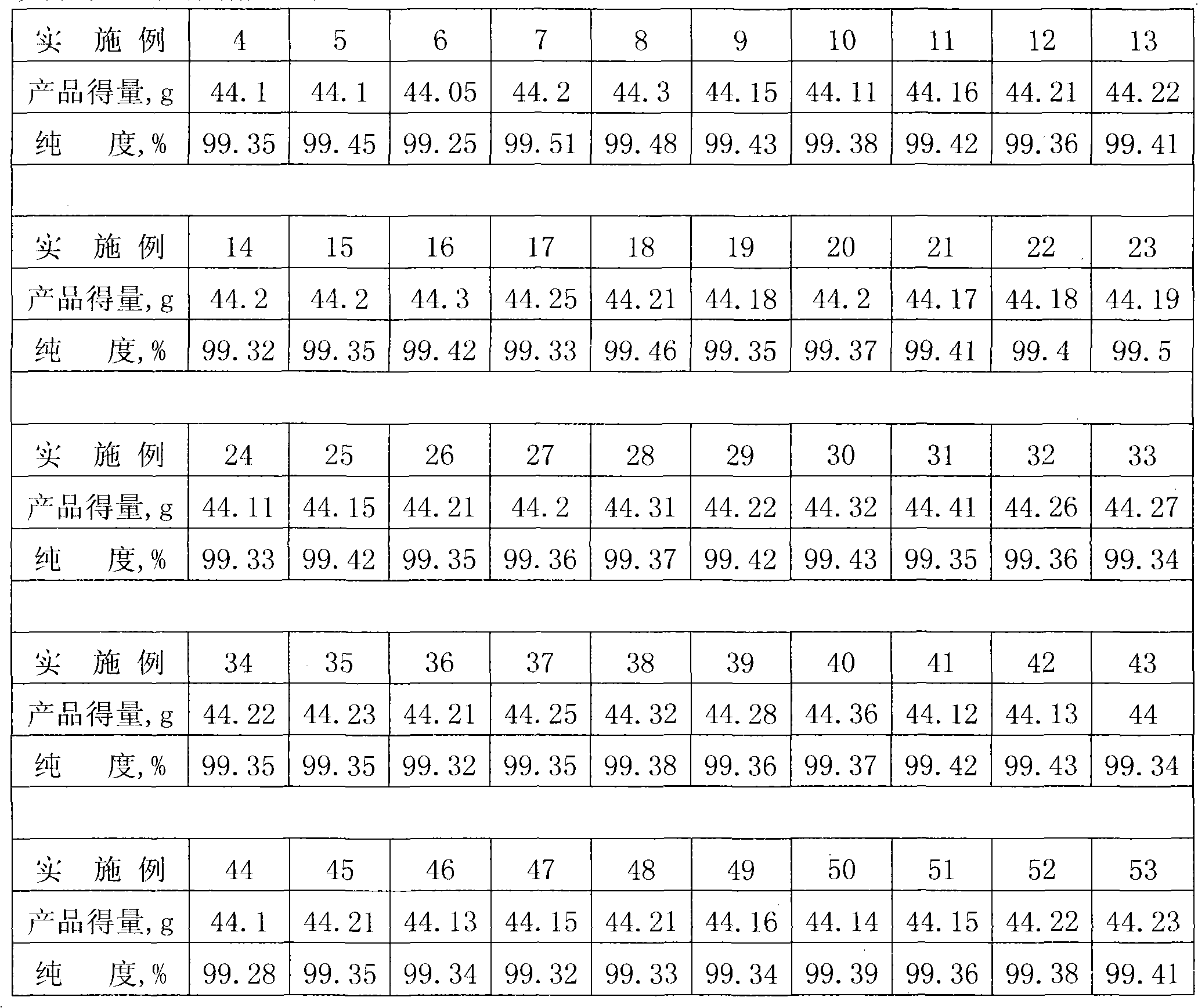Catalyst for reduction of aromatic nitro-compounds by hydrazine hydrate and preparation method thereof
A technology of aromatic nitro group and hydrazine hydrate, applied in the chemical industry, can solve problems such as poor activity and difficult regeneration, and achieve the effects of convenient preparation, resource saving and production cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A catalyst for reducing aromatic nitro compounds with hydrazine hydrate, which comprises the following components in mass percent: 10% of iron, 30% of iron oxide, 35% of ferric oxide and 25% of water.
[0029] The preparation method of the above-mentioned catalyst for reducing aromatic nitro compounds with hydrazine hydrate comprises the following steps:
[0030] (1) In the reactor, add 60ml of water and 20 grams of iron with a content of 93% and a mesh size of 40-60 mesh iron powder, start stirring, and slowly heat up to 80°C;
[0031] (2) heating and reflux reaction at 85°C for 3 hours, and adjusting the pH of the system with hydrochloric acid during the reflux process to be 3-5;
[0032] (3) After the reflux is finished, heat filter, wash with 20ml hot water until neutral, and drain to obtain 30 grams of the catalyst for reducing aromatic nitro compounds with hydrazine hydrate.
[0033] The application process of the catalyst in the reduction of aromatic nitro compo...
Embodiment 2
[0035] A catalyst for reducing aromatic nitro compounds with hydrazine hydrate, which comprises the following components in mass percent: 13% of iron, 33% of iron oxide, 37% of ferric oxide and 17% of water.
[0036] The preparation method of the above-mentioned catalyst for reducing aromatic nitro compounds with hydrazine hydrate comprises the following steps:
[0037] (1) In the reactor, add 60ml of water and 20 grams of iron with a content of 93% and a mesh size of 40-60 mesh iron powder, start stirring, and slowly heat up to 90°C;
[0038] (2) heating and reflux reaction at 90°C for 4 hours, and adjusting the pH of the system with sulfuric acid during the reflux process to be 3-5;
[0039] (3) After the reflux is finished, heat filter, wash with 20ml hot water until neutral, and drain to obtain 30.2 grams of the catalyst for reducing aromatic nitro compounds with hydrazine hydrate.
[0040] The application process of the catalyst in the reduction of aromatic nitro compoun...
Embodiment 3
[0042] A catalyst for reducing aromatic nitro compounds with hydrazine hydrate, which comprises the following components in mass percent: 15% iron, 35% iron oxide, 40% ferric oxide and 10% water.
[0043] The preparation method of the above-mentioned catalyst for reducing aromatic nitro compounds with hydrazine hydrate comprises the following steps:
[0044] (1) In the reactor, add 60ml of water and 20 grams of iron, the content of which is 93% and the mesh size is 40-60 mesh iron powder, start stirring, and slowly heat up to 100°C;
[0045] (2) Heating and reflux reaction at 95° C. for 6 hours, and adjusting the pH of the system with acetic acid during the reflux process to be 3-5;
[0046] (3) After the reflux is completed, heat filter, wash with 20ml hot water until neutral, and drain to obtain 30.1 g of the catalyst for reducing aromatic nitro compounds with hydrazine hydrate.
[0047] The application process of the catalyst in the reduction of aromatic nitro compounds wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com