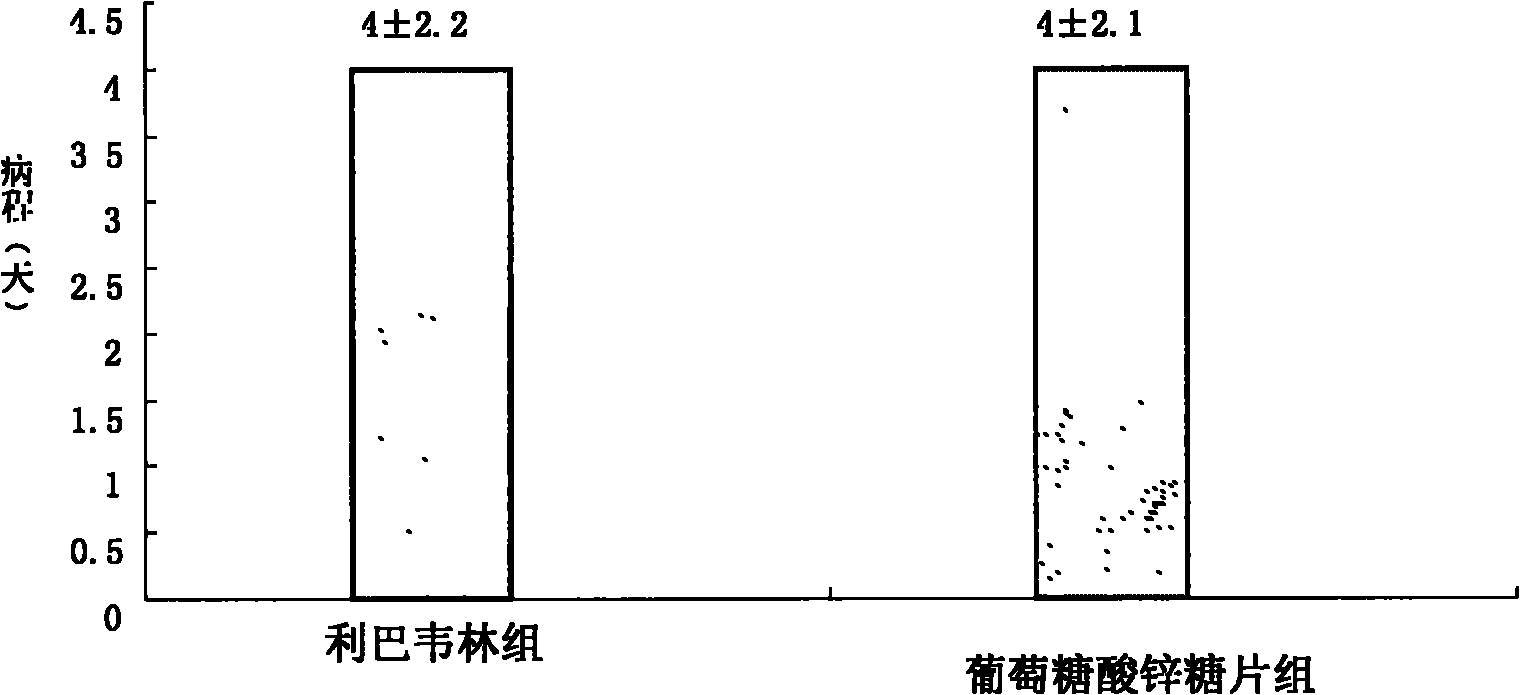
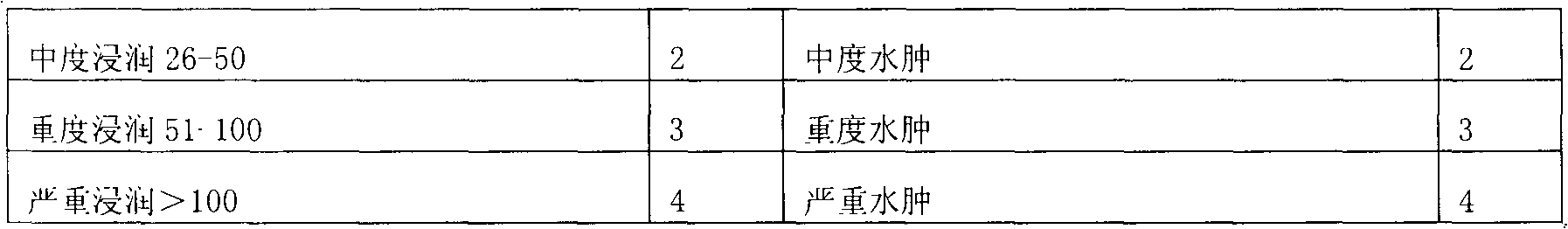
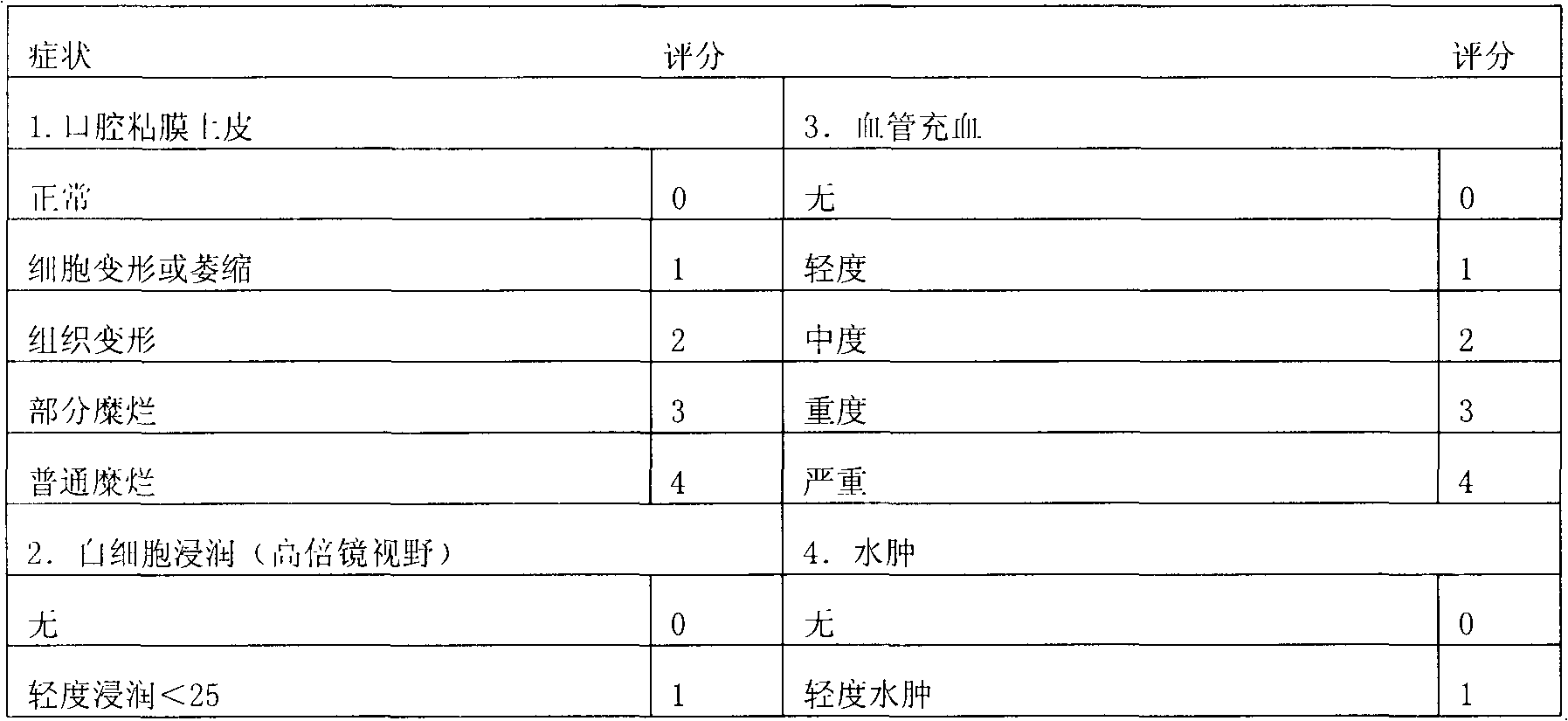Zinc gluconate sugar tablet preparation and preparation method thereof
A technology of zinc gluconate and glucose syrup, applied in the field of medicine, can solve the problems of strong irritation of nasal mucosa, unsatisfactory effect of cold effect, limited clinical application, etc., and achieve the effects of avoiding metallic taste, being happy to take, and controllable quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1. Preparation of zinc gluconate sugar tablets
[0063] 1. The proportion of raw and auxiliary materials:
[0064] Zinc Gluconate: White Sugar: Maltose Syrup: Glycine: Fruit Flavoring Agent: Purified Water = 93.24: 1460: 2910 (in solids): 30: 30: 438 (mass ratio W / W)
[0065] 2. The preparation method, the steps are as follows:
[0066] (1) Weigh 1.865 kg of zinc gluconate, 0.6 kg of glycine, 29.2 kg of white sugar, 58.2 kg of maltose syrup (solids), 8.76 kg of purified water, and 0.6 kg of fruit flavoring agent according to the prescription.
[0067] (2) Add the weighed white sugar, maltose syrup and purified water into the dissolving tank, turn on the stirring, heat to 80-90°C to completely dissolve it; put in spare zinc gluconate and glycine, and continue stirring for 10 minutes. After completely dissolved. Turn on the vacuum pump, and pump the completely dissolved sugar liquid into the vacuum sugar evaporator (preheated at 90-100°C).
[0068] (3) Turn on the circulat...
Embodiment 2
[0073] Example 2. Preparation of zinc gluconate sugar tablets
[0074] 1. The proportion of raw and auxiliary materials:
[0075] Zinc Gluconate: White Sugar: Maltose Syrup: Glycine: Fruit Flavoring Agent: Purified Water = 93.24:1880:3750 (solid matter): 39:39:565 (mass ratio W / W)
[0076] 2. The preparation method, the steps are as follows:
[0077] 1) Weigh 1.865 kg of zinc gluconate, 0.78 kg of glycine, 37.6 kg of white sugar, 75 kg of maltose syrup (solids), 11.3 kg of purified water and 0.78 kg of fruit flavoring agent according to the prescription amount.
[0078] 2) Add the weighed white sugar, maltose syrup and purified water into the dissolving tank, turn on the stirring, heat to 80~90℃, and dissolve completely; put in spare zinc gluconate and glycine, continue to stir for 10 minutes, complete After dissolving; add flavoring fruit flavoring agent. Turn on the vacuum pump, and pump the completely dissolved sugar liquid into the vacuum sugar evaporator (preheated at 90-100°C). ...
Embodiment 3
[0083] Example 3. Preparation of zinc gluconate sugar tablets
[0084] 1. The proportion of raw and auxiliary materials:
[0085] Zinc Gluconate: White Sugar: Glucose Syrup: Glycine: Fruit Flavoring Agent: Purified Water = 93.24:1670:3330 (solid matter): 35:35:502 (mass ratio W / W)
[0086] 2. The preparation method, the steps are as follows:
[0087] (1) Weigh 1.865 kg of zinc gluconate, 0.7 kg of glycine, 33.4 kg of sugar, 66.6 kg of glucose syrup (solids), 10 kg of purified water and 0.7 kg of fruit flavoring agent according to the prescription amount.
[0088] The operations of (2) to (4) are the same as the corresponding steps (2) to (4) of Example 1.
[0089] (5) Control the temperature of the kneaded and uniform massecuite at 70-80℃, and send it to the heat-preserving rolling machine to roll into strips, and then enter the homogenizer to draw uniform and suitable sugar bars and enter the punching machine. Adjust the pressure of the punching machine and the tablet weight to 5.2 g / t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com