Insulin B chain HLA-A*0201 restrictive CTL epitope modified peptide ligand and acquisition method and application thereof
A technology for HLA-A and insulin, applied in the preparation methods of peptides, pharmaceutical formulations, chemical instruments and methods, etc., can solve the problems of difficult to grasp the scale of immune response type conversion, unpredictable final outcome of diseases, etc., and achieve easy scale. The effect of preparation and purification, reducing research costs, and safe use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] Hereinafter, preferred embodiments of the present invention will be described in detail with reference to the accompanying drawings.
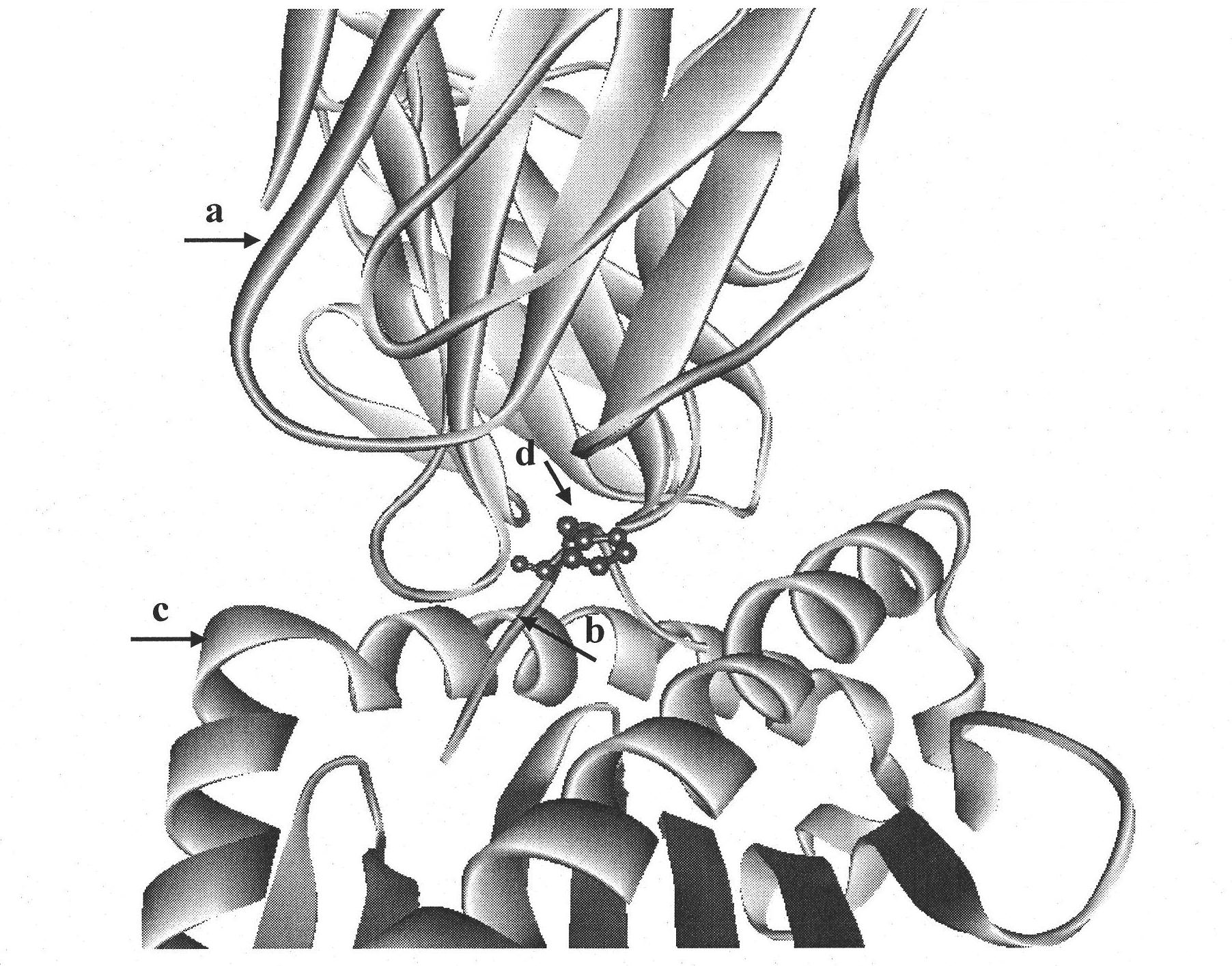
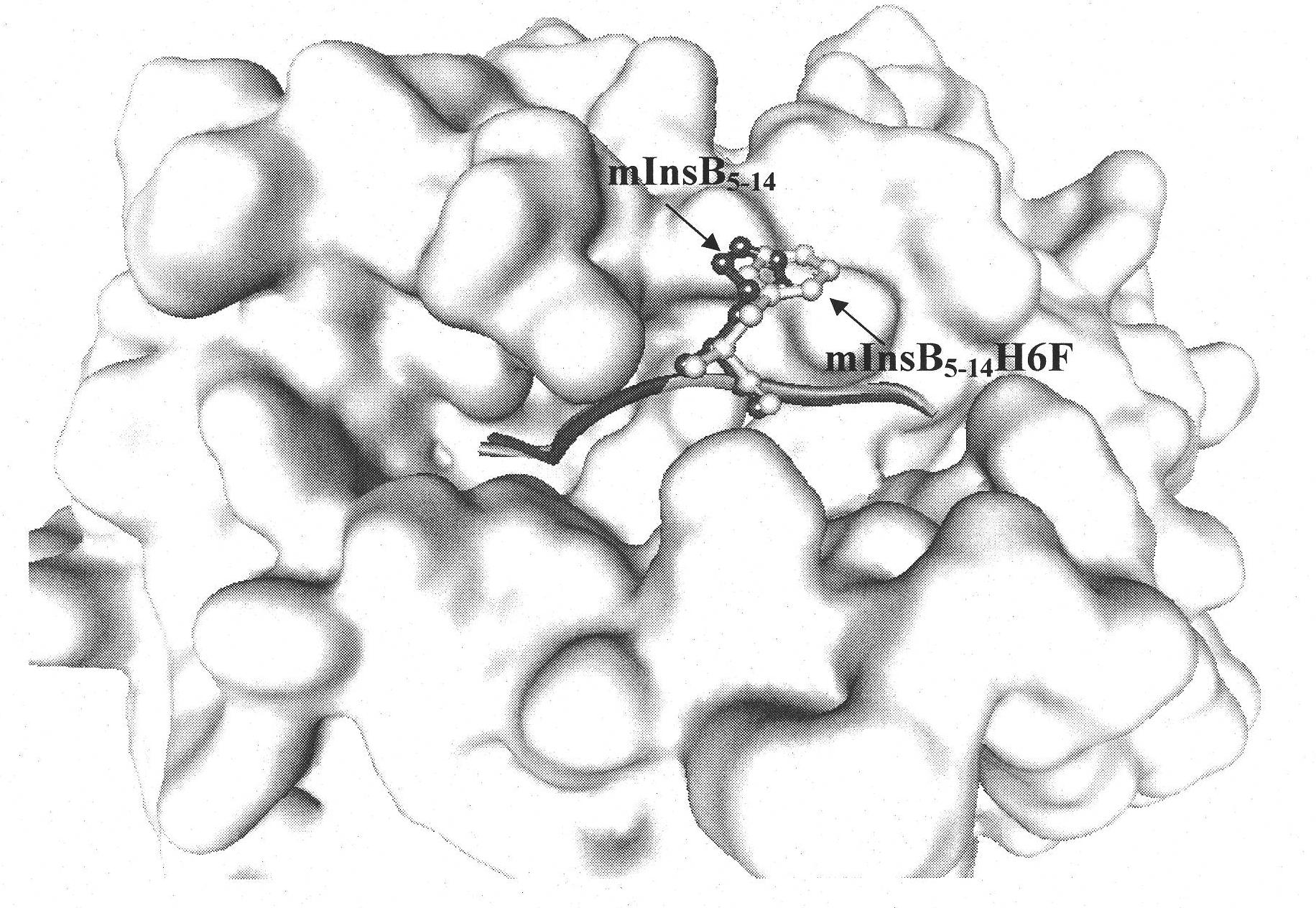
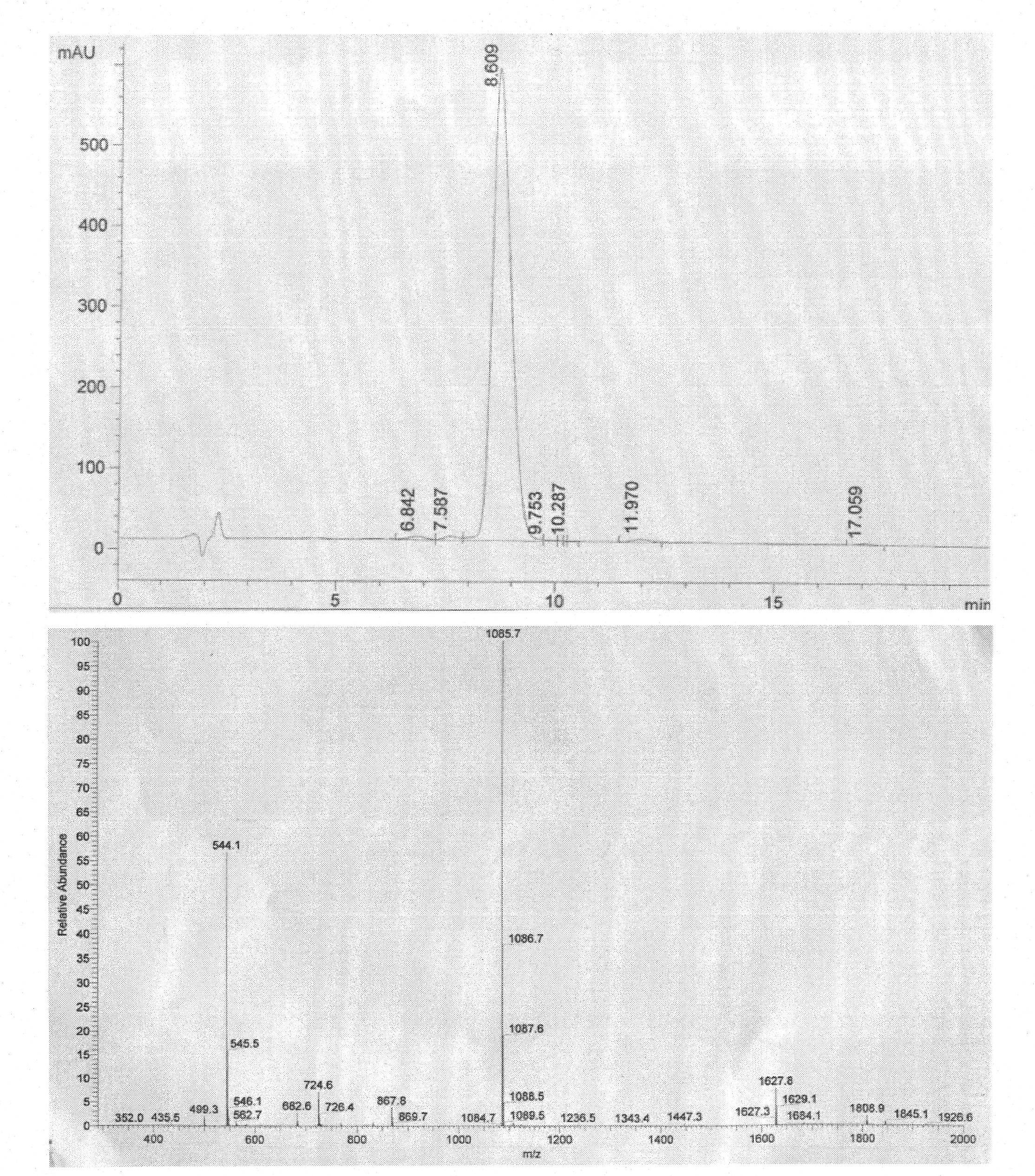
[0025]1. Establishment of TCR-HLA-A*0201-mInsB 5-14 A structural model of the ternary complex identifying the native epitope mInsB 5-14 Potential TCR main action site in
[0026] Using Insight II software to first establish the natural epitope mInsB 5-14 (amino acid sequence is HLCGPHLVEA) and HLA-A*0201 binary complex (pMHC) structure model, and then further establish the ternary complex structure model of pMHC and TCR ( figure 1 ), analyzing the natural epitope mInsB 5-14 Amino acid residues that may interact with TCR. The results showed that the natural epitope mInsB 5-14 The 5th position and the 6th position in both are potential TCR main action sites, but the proline residue at the 5th position has a greater impact on the conformation of the polypeptide, so the present invention only uses the 6th position as Modification site....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com