Cell type used for producing induced pluripotent stem (iPS) cells and preparation method and application thereof
A technology for pluripotent stem cells and pluripotent stem cells, which is applied in the field of cell types and their preparation, and can solve the problems of low efficiency of iPS cells, low efficiency of iPS, and carrier retention.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Cell Preparation and Culture
[0056] For amniocytes, a certain amount of amniotic fluid is obtained by amniocentesis of pregnant women undergoing prenatal care. The precipitate obtained after centrifugation was resuspended in the amniotic fluid medium for culture for 3-4 days, and then replaced with fresh amniotic fluid medium to continue the culture.
[0057] For the three kinds of cells derived from placenta, the placental tissue and umbilical cord isolated from normal full-term cesarean section waste were taken under sterile conditions, washed several times with 1×PBS, and the amnion and chorion were peeled off with tweezers and placed in PBS separately Then use surgical scissors to cut the amnion and chorion into about 1mm 3small organizational blocks. (1) In order to obtain amniotic mesenchymal cells, trypsin was added to amniotic membrane fragments to digest at 37°C for 30-45 minutes, a total of 3 times, then added Dispase at a final concentration of 1.2U / ml, d...
Embodiment 2
[0062] Viral vectors infect three types of cells derived from placental tissue and amniocytes
[0063] According to the method described in Example 1, in the p6 well culture plate, approximately 4 × 10 4 Each cell was inoculated with three kinds of cells derived from human placenta, and about 8×10 cells per well for amniocytes 4 Cells were seeded at 37°C, 5% CO 2 Cultured overnight under normal culture conditions (such as figure 1 ), and the collected virus supernatants were used to infect three cultured placenta-derived cells and amniocytes respectively. The viral supernatant was obtained by transfecting 293T cells (Lipofectamine 2000, Invitrogen Corporation) with a retroviral pMX vector (Addgene Corporation) containing human Oct4, Sox2, Klf4 and c-Myc cDNA according to conventional methods ( See Sambrook, Fritsch and Maniatis, A Laboratory Guide to Molecular Cloning, 3rd Edition (2002)).
Embodiment 3
[0065] Continued culture of infected cells and clone selection
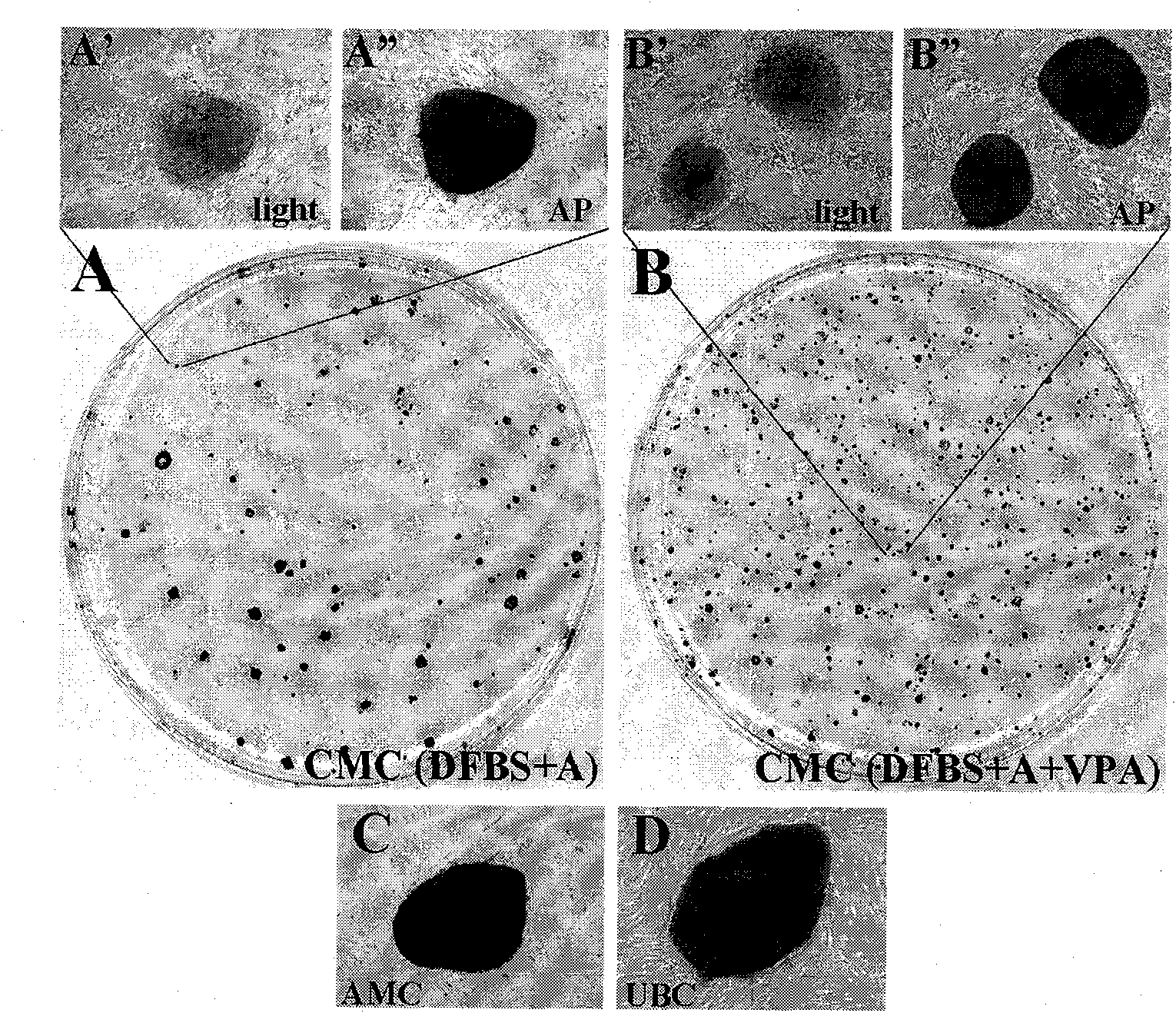
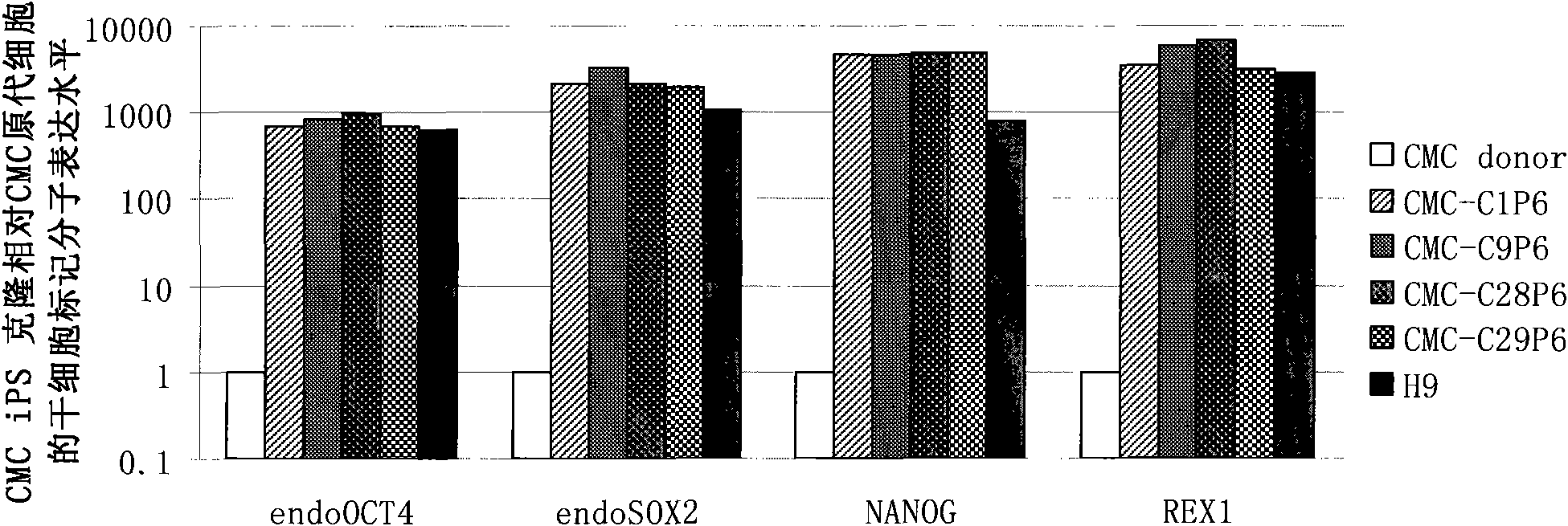
[0066] On the second day after infection, the culture medium of the three kinds of placenta-derived cells and amniocytes after infection was replaced with the DFBS medium described in Example 1, and the 2 Cultivate for 4 days under normal culture conditions, replace the medium once a day, digest it into a single cell suspension with 0.25% trypsin-EDTA (Gibco) at 37°C, and divide the digested cells at a density of about 10,000 cells per culture dish Inoculated into 100 mm culture dishes, which were pre-coated with trophoblast cells as described in Example 1. The seeded cells were incubated at 37°C, 5% CO 2 Under conventional culture conditions, cultured between 15-30 days, ES-like clones can be seen under microscope (such as figure 2 Shown are chorionic mesenchymal cells forming typical hES-like clonal morphology on trophoblast 28 days after infection with SKOM). The hES-like clones were picked and propagated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com