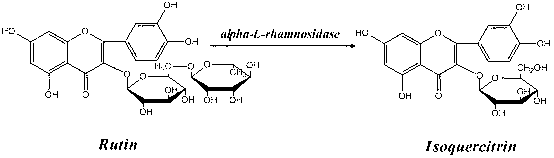
Application of alpha-L-rhamnoside enzyme in directional synthesis of isoquercitrin by biological conversion of rutin
A technology of rhamnosidase and isoquercitrin, which is applied to the direction of immobilization on/in it, fermentation, etc., can solve the problem of high-selectivity and directional preparation of isoquercitrin and rutin orientation. Hydrolysis to prepare isoquercitrin and other problems, to achieve good industrial application prospects, high catalytic efficiency and specificity, and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Selection of strains: Select Aspergillus niger CGMCC No.2588 ( Aspergillus niger LJ-1) as a strain producing α-L-rhamnosidase;
[0031] (2) Preparation of crude enzyme solution of α-L-rhamnosidase:
[0032] Enzyme production medium formula (weight ratio): 0.1% potassium hydrogen phosphate, 0.1% potassium dihydrogen phosphate, 0.1% ammonium sulfate, 0.01% sodium nitrate, 0.01% magnesium sulfate, 0.001% ferrous sulfate heptahydrate, zinc sulfate 0.001%, sucrose 1%, pH 5.0.
[0033] The strains were inoculated in the α-L-rhamnosidase enzyme production medium with a conventional amount, and fermented for 120 hours at 45°C with a shaker speed of 100 rpm, and then all the fermented products were mixed at a speed of 8000 rpm Centrifuge for 10 minutes, collect the supernatant to obtain the crude enzyme solution of α-L-rhamnosidase;
[0034] (3) α-L-rhamnosidase catalyzes the directional biosynthesis of isoquercitrin by hydrolyzing rutin:
[0035] A. The free enzyme of ...
Embodiment 2
[0042] (1) Selection of strains: Select Penicillium CGMCC No. 2590 ( Penicillium notatum LJ-2) as a strain producing α-L-rhamnosidase;
[0043] (2) Preparation of crude enzyme solution of α-L-rhamnosidase:
[0044] Enzyme production medium formula (weight ratio): 1.5% potassium dihydrogen phosphate, 1.5% potassium dihydrogen phosphate, 1.5% ammonium sulfate, 0.5% sodium nitrate, 0.5% magnesium sulfate, 0.5% ferrous sulfate heptahydrate, zinc sulfate 0.5%, sucrose 5%, pH 8.0.
[0045] The strains were inoculated in the α-L-rhamnosidase enzyme production medium in a conventional amount, and fermented for 12 hours at 25°C with a shaker rotation speed of 250 rpm, and then all the fermented products were mixed at a rotation speed of 4000 rpm Centrifuge for 30 minutes, collect the supernatant to obtain the crude enzyme solution of α-L-rhamnosidase;
[0046] (3) α-L-rhamnosidase catalyzes the directional biosynthesis of isoquercitrin by hydrolyzing rutin:
[0047] A. The free e...
Embodiment 3
[0054] (1) Selection of strains: Select Aspergillus niger CGMCC No.2588 ( Aspergillus niger LJ-1) as a strain producing α-L-rhamnosidase;
[0055] (2) Preparation of crude enzyme solution of α-L-rhamnosidase:
[0056] Enzyme production medium formula is (weight ratio): dipotassium hydrogen phosphate 1.0%, potassium dihydrogen phosphate 0.5%, ammonium sulfate 0.5%, sodium nitrate 0.05%, magnesium sulfate 0.05%, ferrous sulfate 0.01%, zinc sulfate 0.01% , pH 6.0.
[0057] The strains were inoculated in the α-L-rhamnosidase enzyme production medium in a regular amount, and fermented for 72 hours at 35°C with a shaker rotation speed of 160 rpm, and then all the fermented products were mixed at a rotation speed of 5000 rpm Centrifuge for 20 minutes, collect the supernatant to obtain the crude enzyme solution of α-L-rhamnosidase;
[0058] (3) α-L-rhamnosidase catalyzes the directional biosynthesis of isoquercitrin by hydrolyzing rutin:
[0059] A. The free enzyme of α-L-rhamnos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com