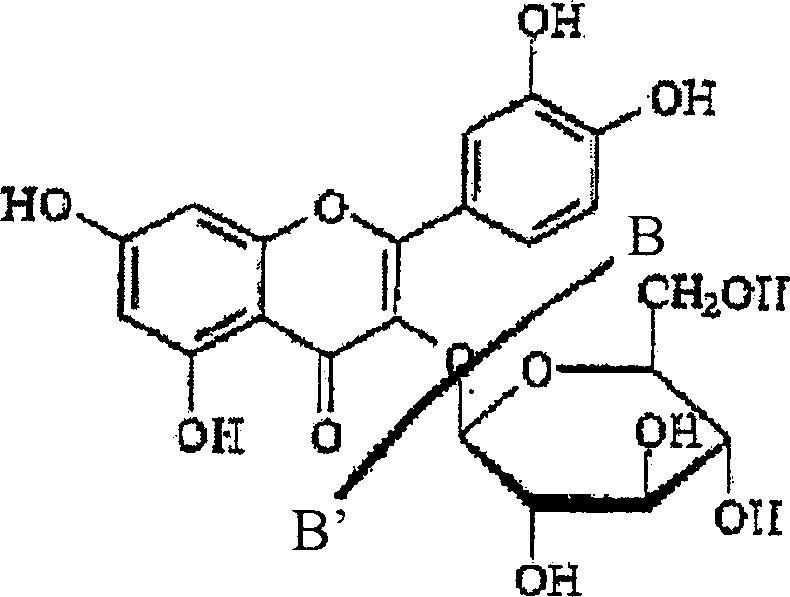
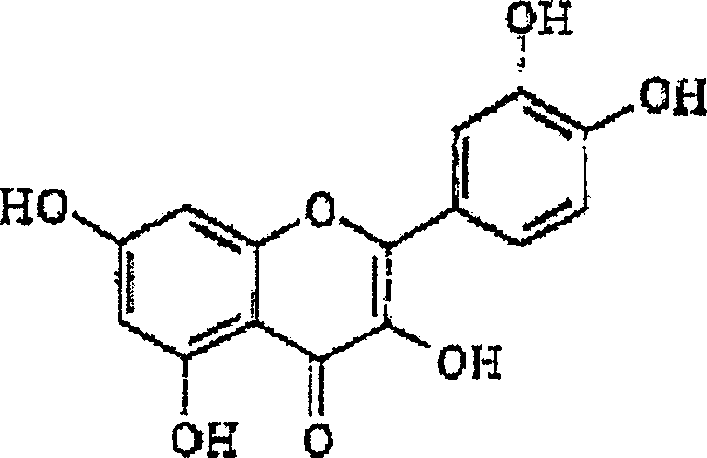
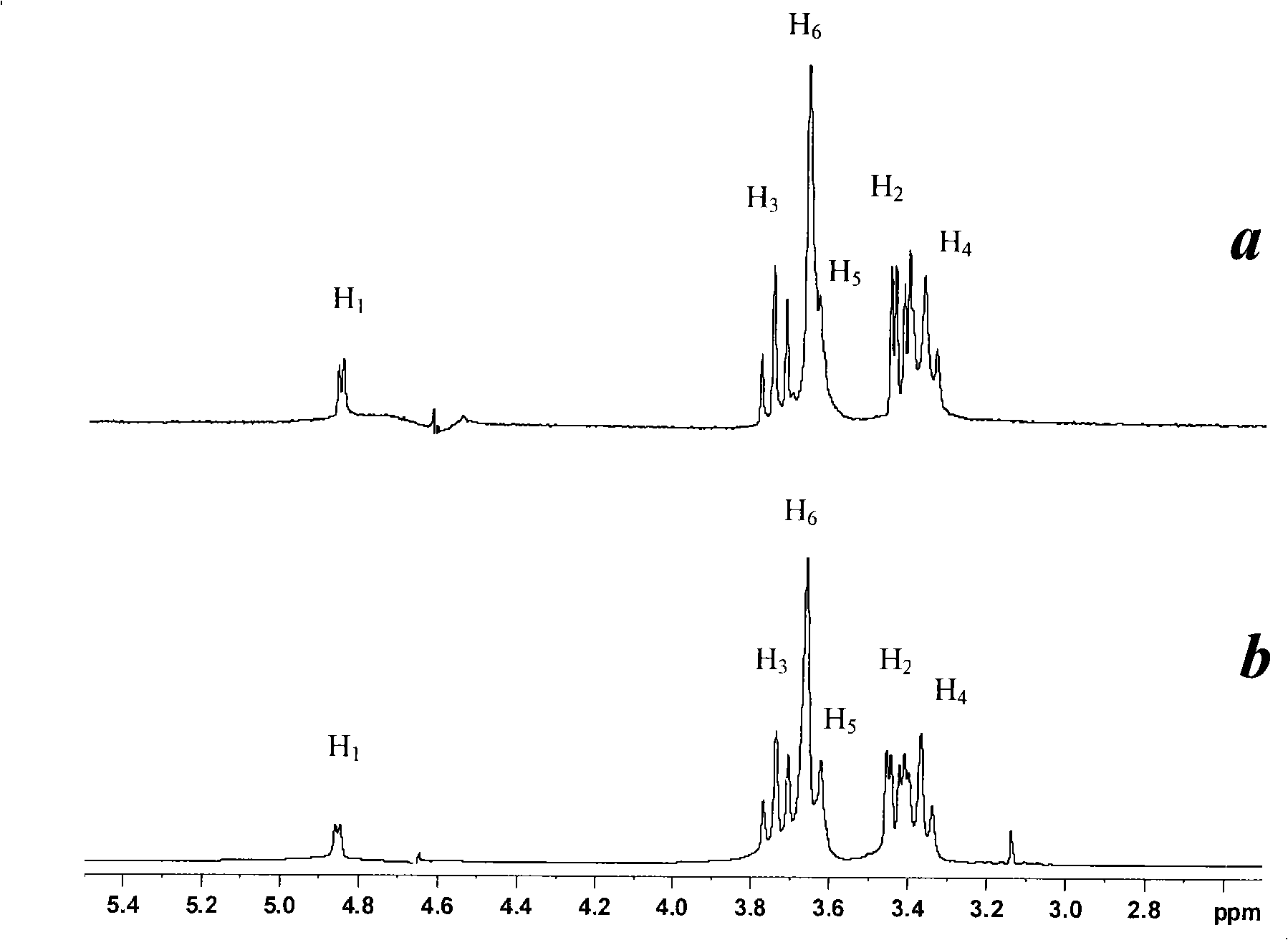
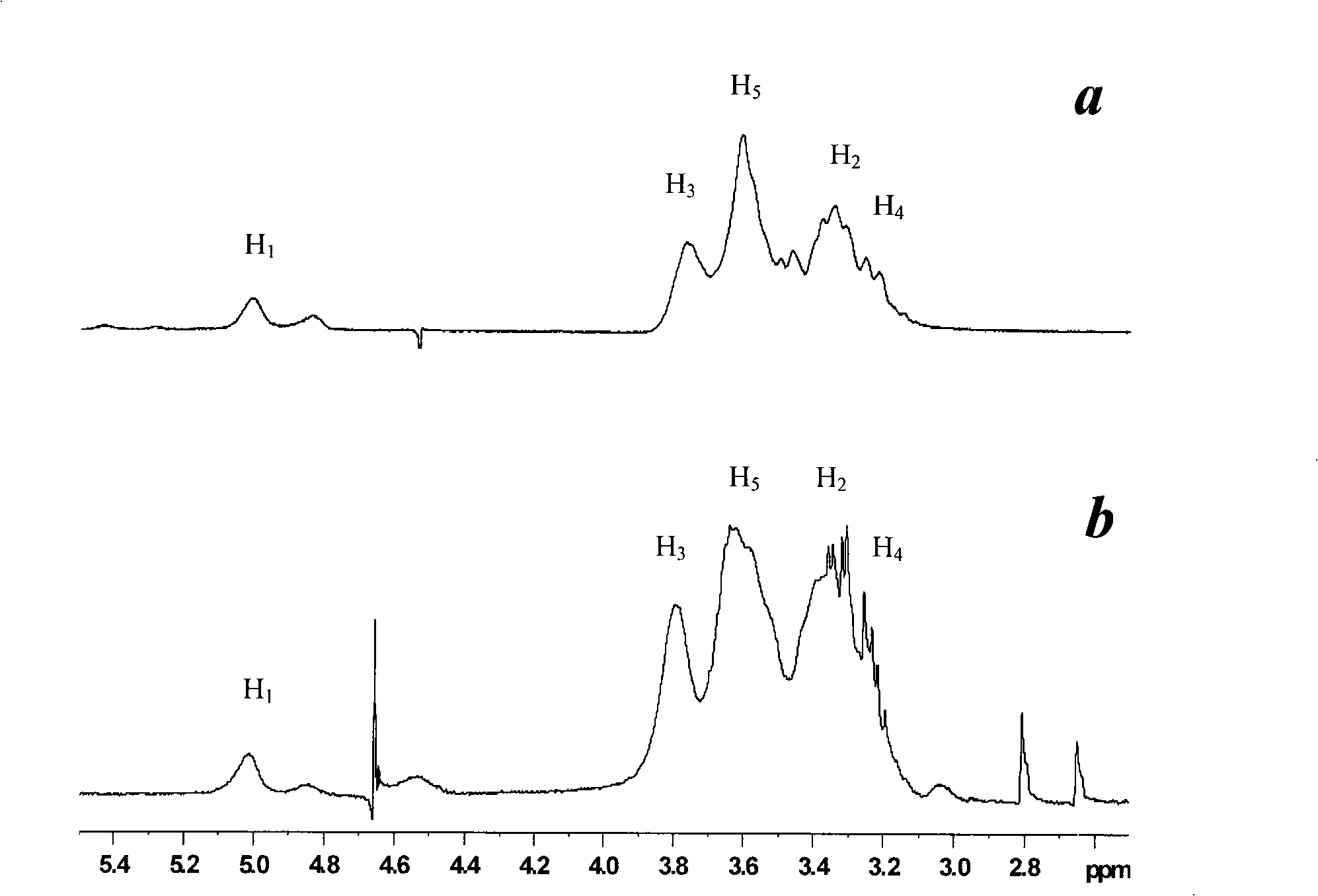
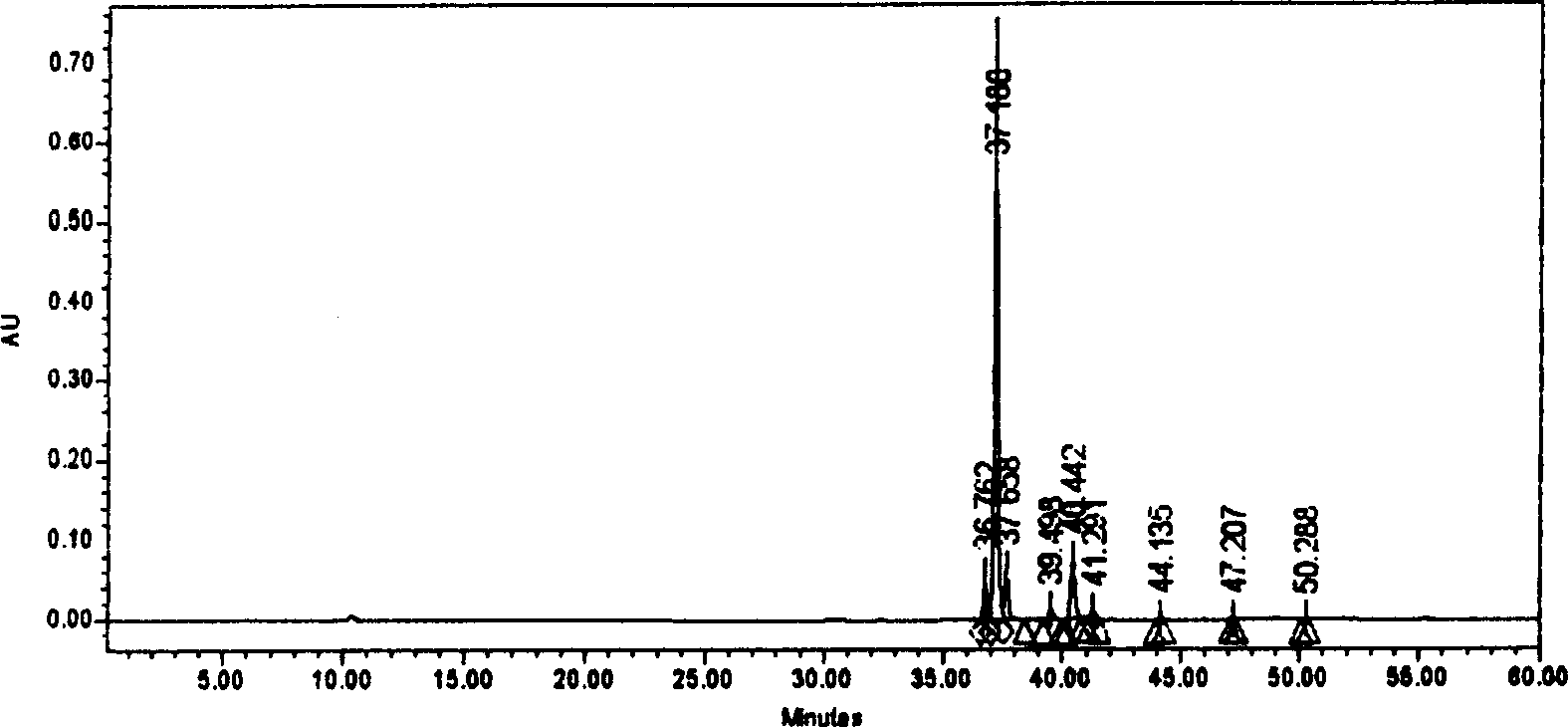
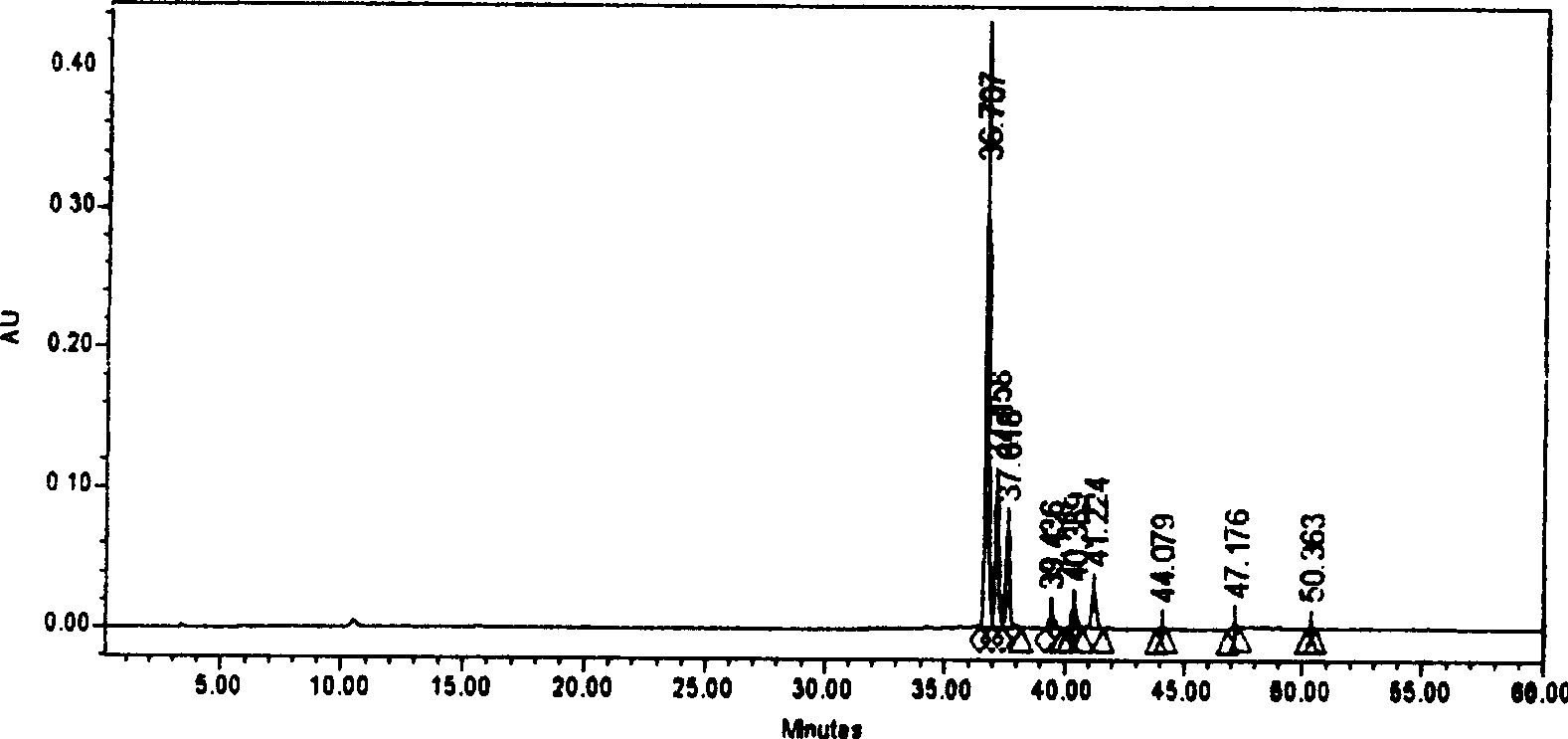
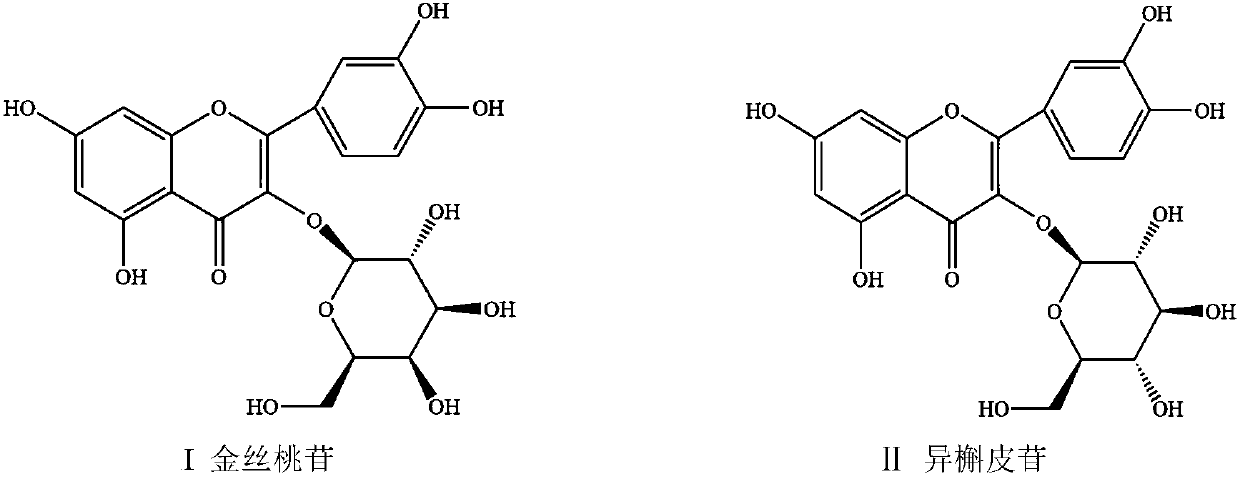
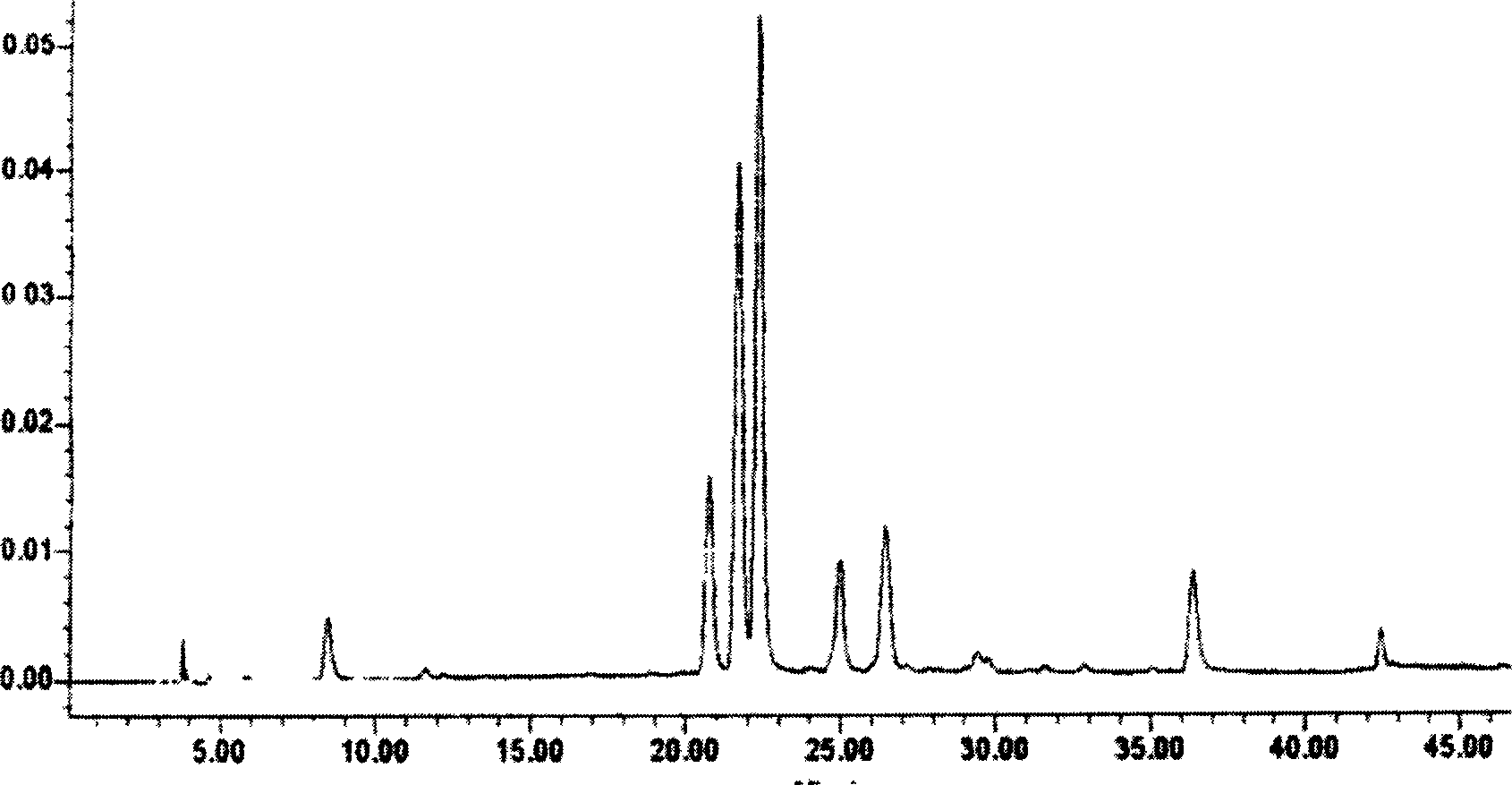
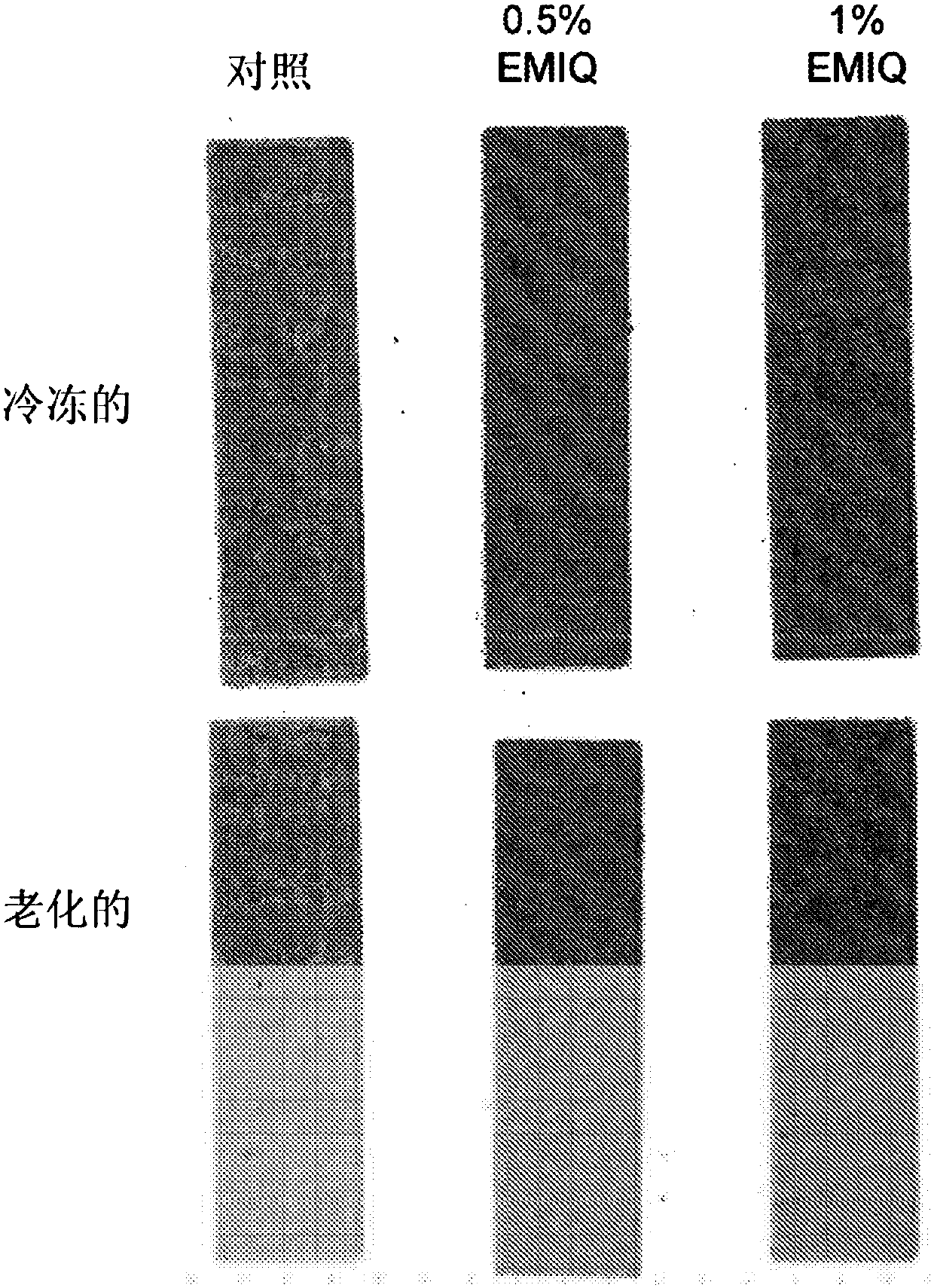
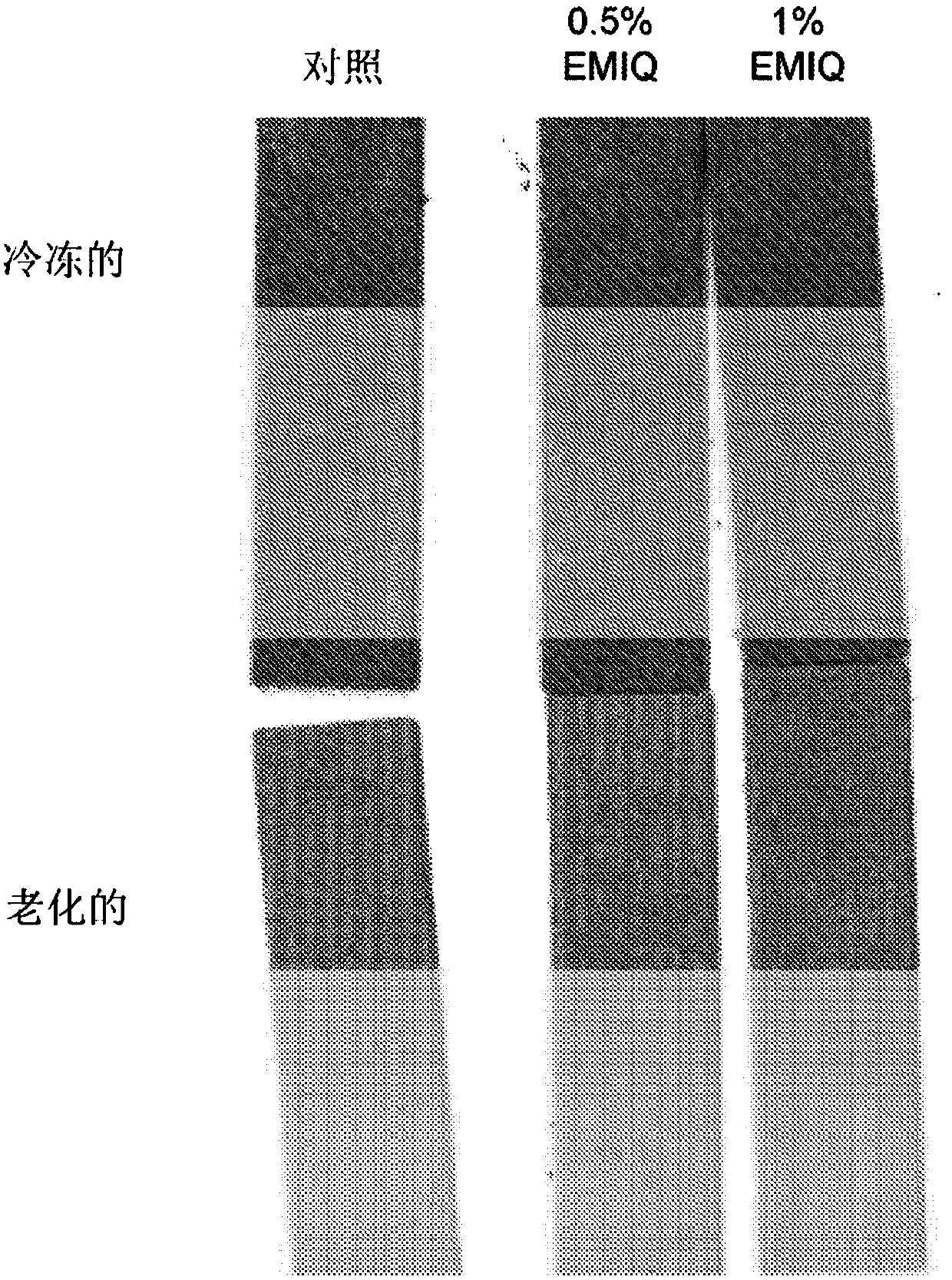
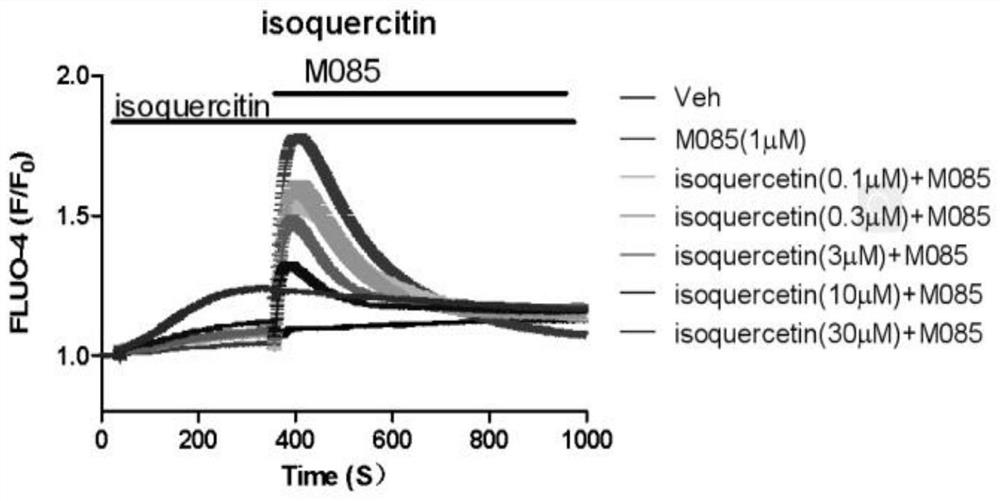
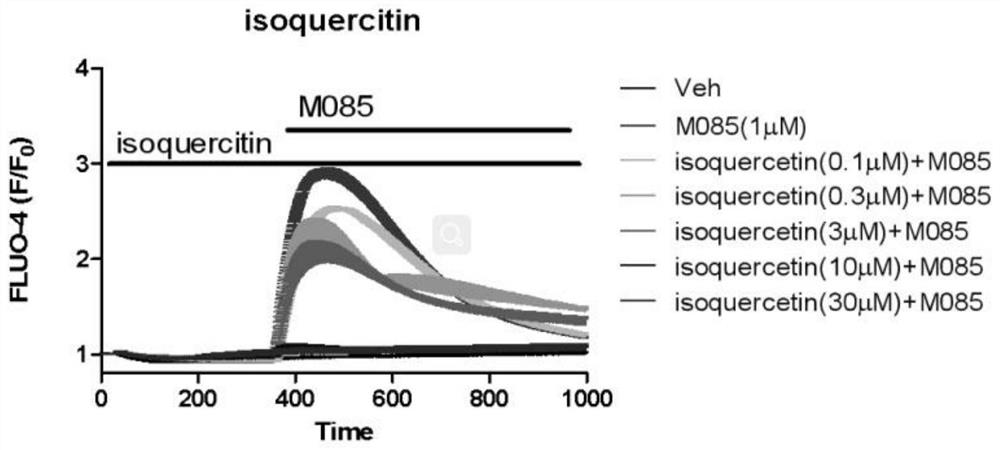
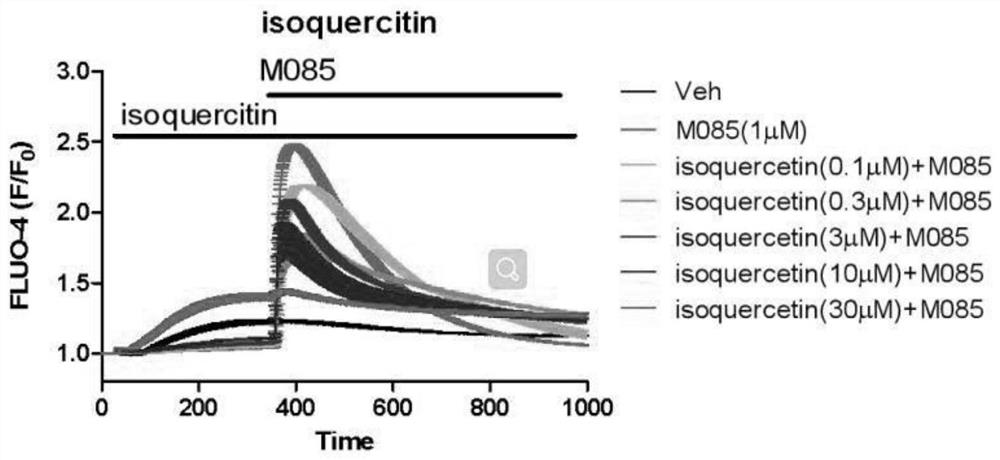
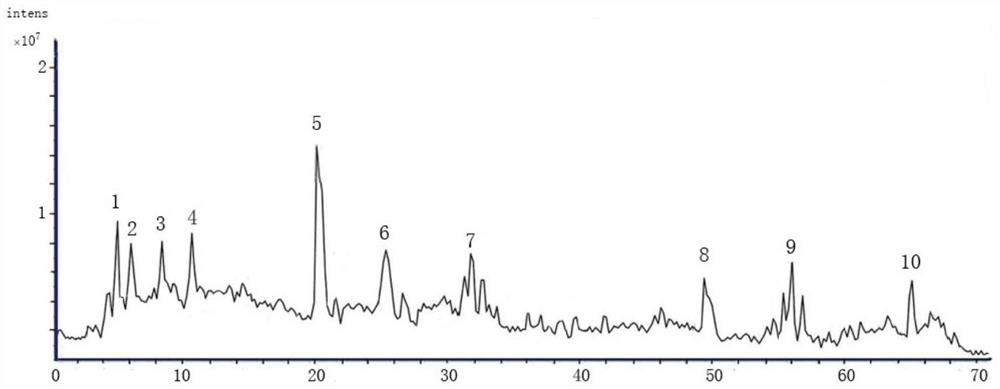
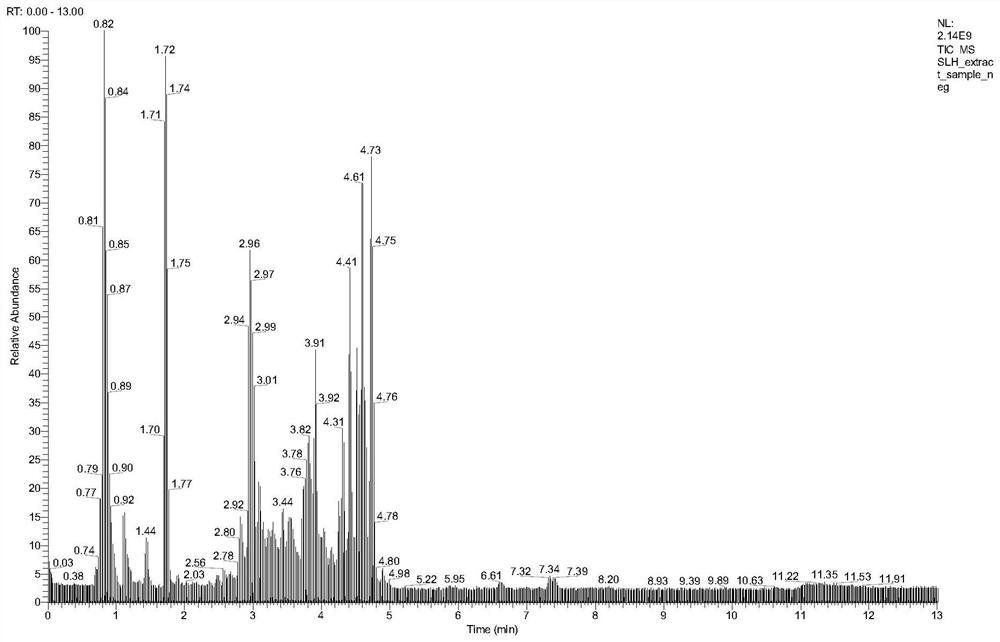
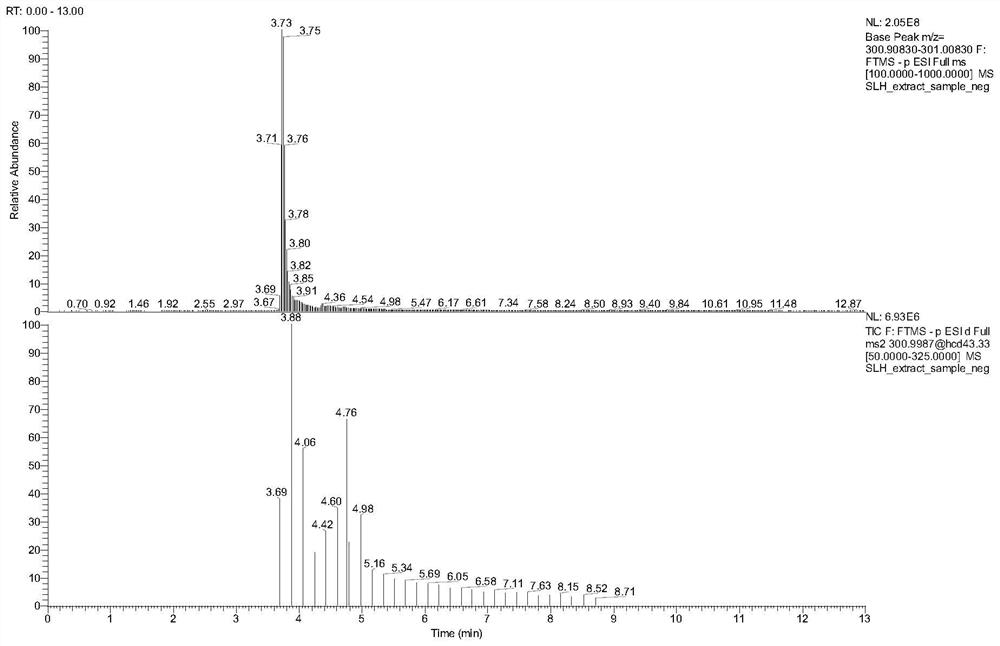
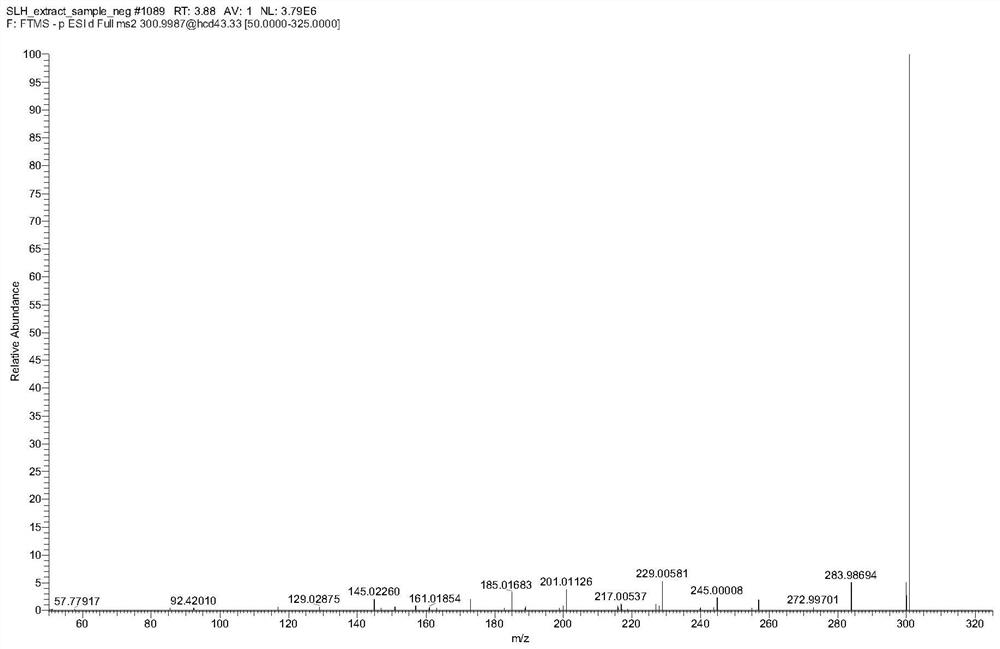
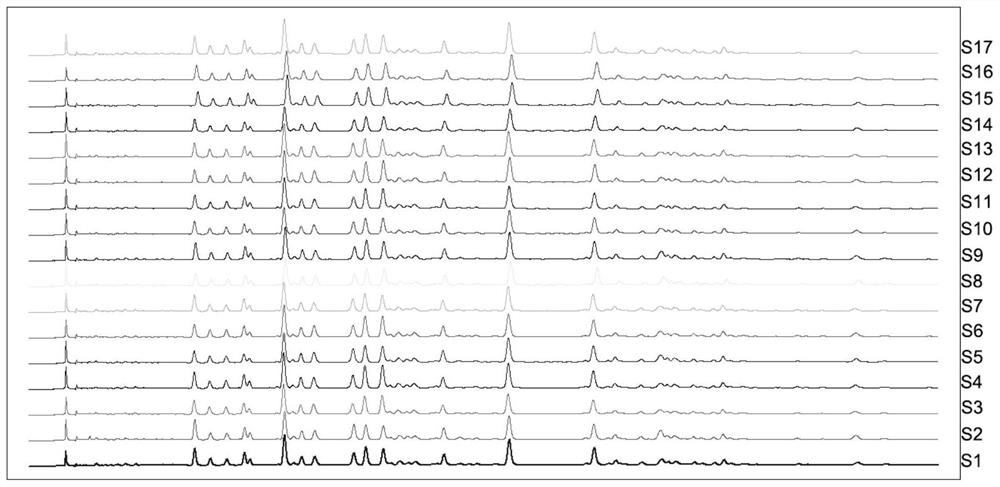
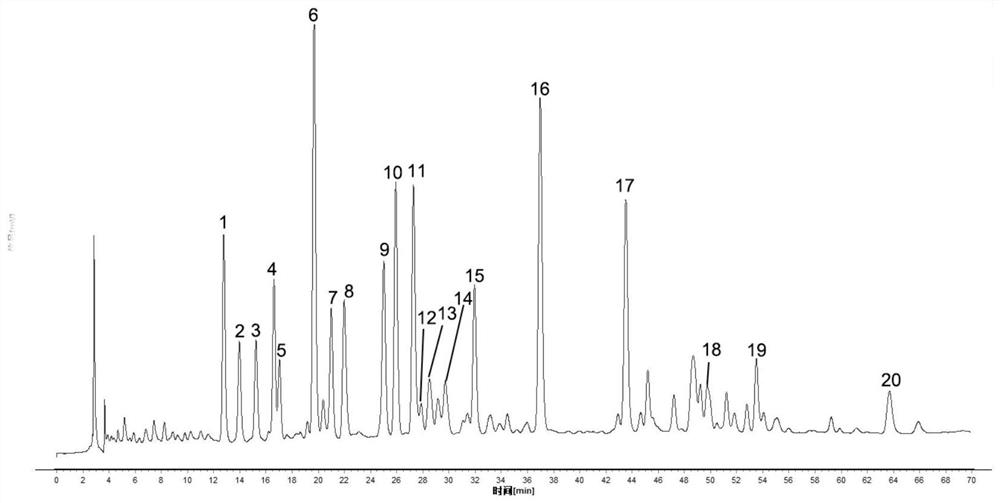
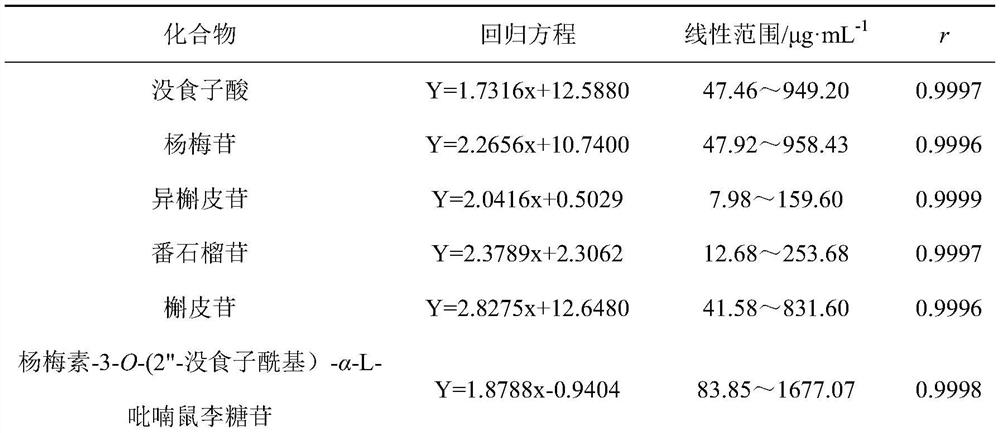
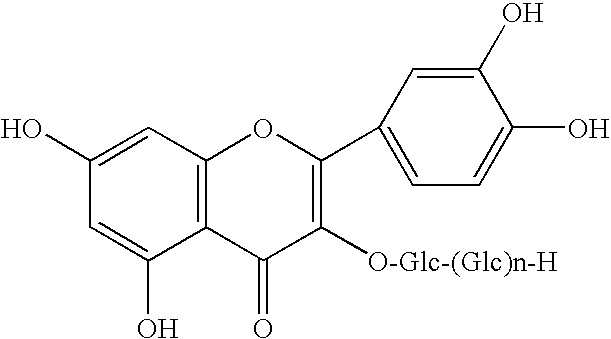
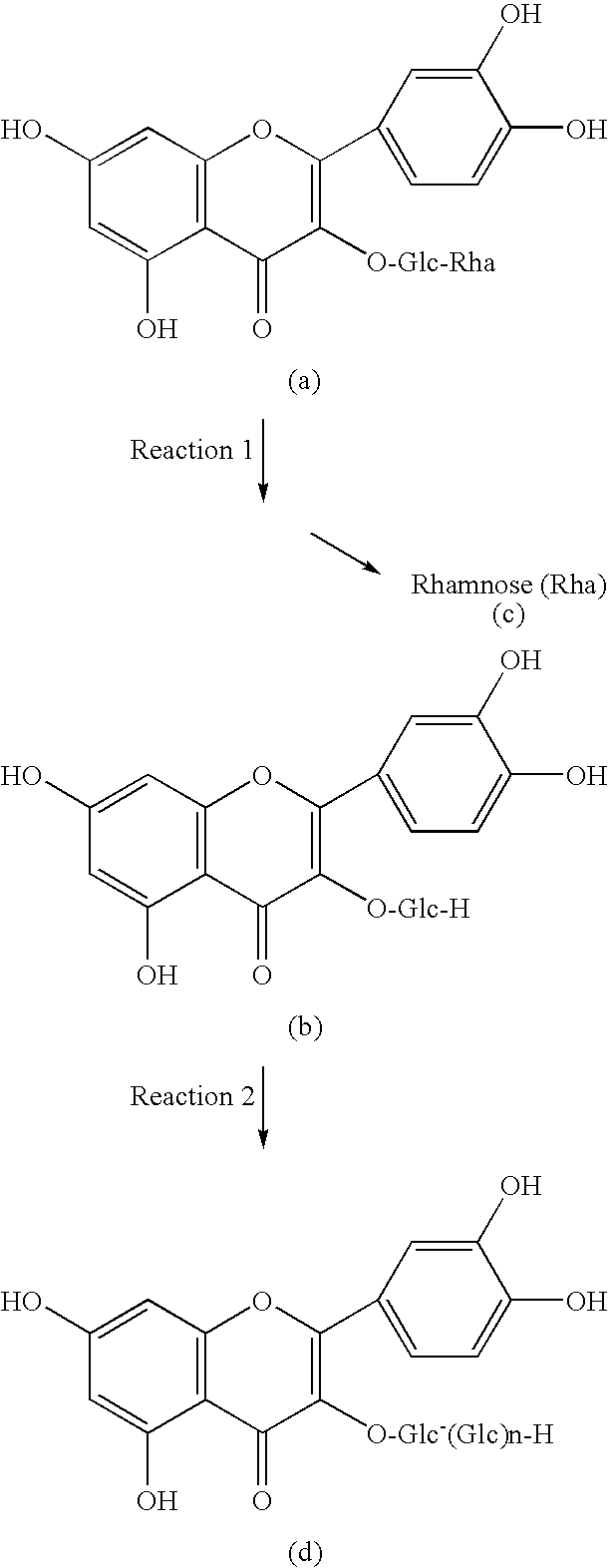
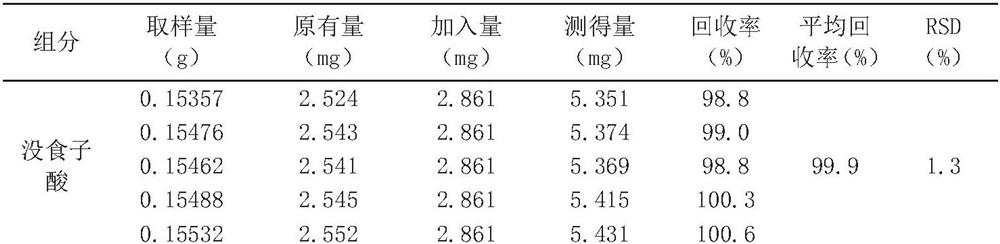
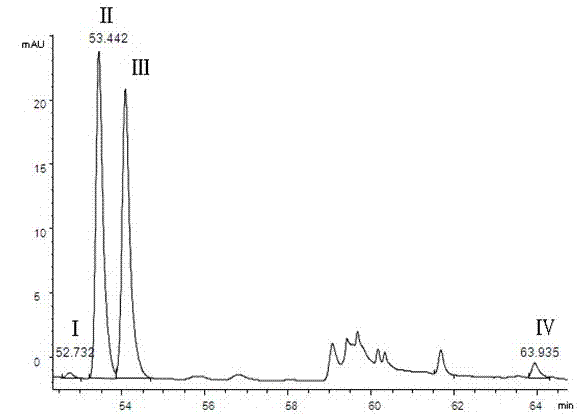
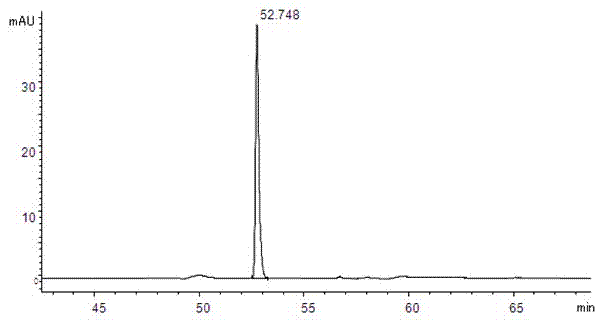
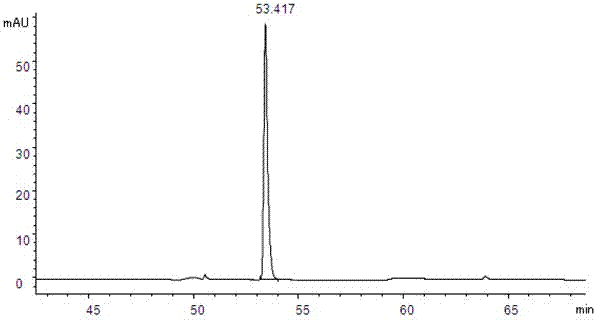
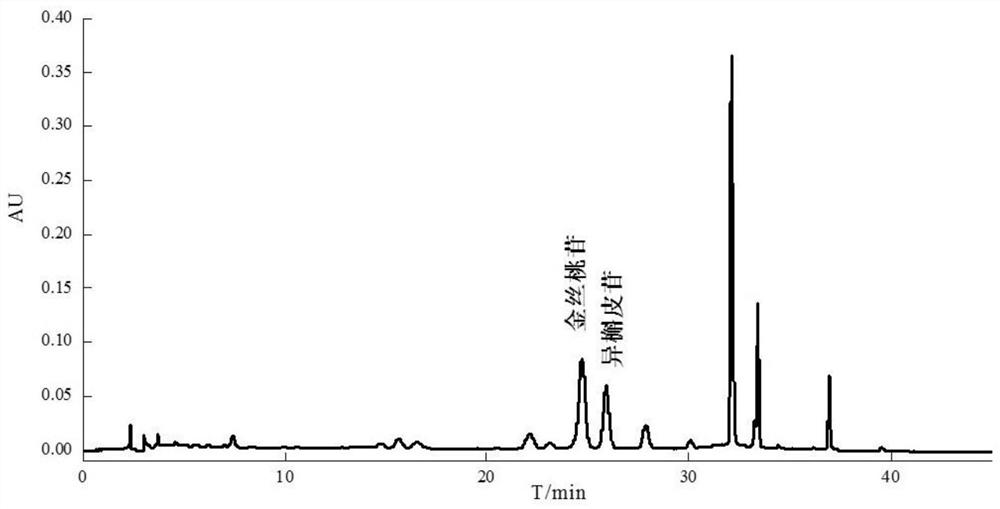
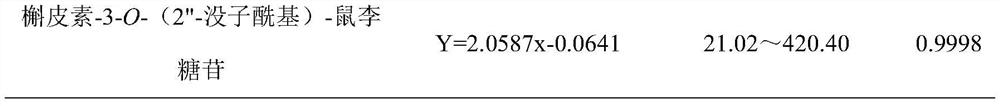Patents
Literature
54 results about "Isoquercitroside" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
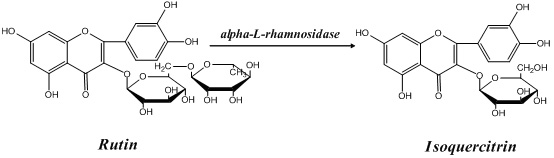
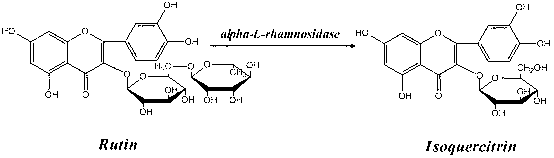
Application of alpha-L-rhamnoside enzyme in directional synthesis of isoquercitrin by biological conversion of rutin
ActiveCN101985639AImprove catalytic conversionLow costOn/in organic carrierFermentationPtru catalystEnzyme catalysis
The invention relates to application of an alpha-L-rhamnoside enzyme in the directional synthesis of isoquercitrin by the biological conversion of rutin. Because the crude enzyme preparation or the immobilized enzyme preparation of the alpha-L-rhamnoside enzyme is used for catalyzing and hydrolyzing the rutin and directionally and biologically synthesizing the isoquercitrin, the application has the advantages of wide source, easy preparation and low cost of catalysts, high stability, and high catalysis efficiency and specificity of the enzyme preparation, and is easy to store. Thus, the alpha-L-rhamnoside enzyme can greatly reduce the production cost of quercetin, the catalysis and conversion rate of the enzymes is high, and the products are single.
Owner:JIANGSU UNIV OF SCI & TECH
Extraction, purification and conversion of flavonoids from plant biomass
InactiveCN1685053AEffective biotransformationOrganic active ingredientsCosmetic preparationsRutinOrganic chemistry
A process for preparing a rutin-enriched composition from plant biomass comprises extraction with an aqueous solution, and precipitation. An enzyme preparation, such as naringinase, is used for the transformation of rutin to higher value compositions containing increased proportions of isoquercitrin and quercetin.
Owner:加拿大农业部
Maniod ebish flower extract, extraction and analysis method and extract preparation and use thereof
InactiveCN101385748AGood treatment effectNotable mechanism of actionPill deliveryCapsule deliveryDiseaseAdditive ingredient
An extract from Sunset Abelmoschus, containing 50-75wt% of total flavone counted by hyperin, wherein, the contents of hyperin, isoquercitrin and quercetin-3'-glucoside are 10.0-15.0wt%, 7.0-12.0wt% and 8.0-12.0wt% respectively, and a total flavone finger-print is provided. The extract is obtained by the following steps: a concentrated solution of a Sunset Abelmoschus ethanol extract undergoes chromatography through an HPD400 or HPD700 macroporous absorbent resin column to derive a crude extract or is extracted by normal butanol to derive the crude extract; a refined extract is obtained by refluxing extraction with ethyl acetate-ethanol azeotropic mixture or ethyl acetate-methanol mixture; the refined extract goes through pressure reducing exsolution and drying. Corresponding contents and a method for detecting finger-print are also provided. The extract preparation is a dripping pill, tablet, capsule or soft capsule obtained through processing with the extract as an active ingredient and pharmaceutical excipients. The extract can be used for preparing a medicine preparation for treating cardiac anencephalohemia diseases.
Owner:周春晓 +1
Kindir leaf total flavone extract and its prepn and application
InactiveCN1931205ASignificant anti-ischemic effectClear certaintyOrganic active ingredientsSugar derivativesTrifolinAstragaloside
The present invention is kinder leaf total flavone extract and its preparation process and application, and features that the kinder leaf total flavone extract contains total flavone in 50-95 %, including rutin 0.2-30.0 %, hyperin 0.5-30.0 %, isoquercitrin 10.0-30.0 %, trifolin 3.0-20.0 %, astragaloside 2.0-5.0 %, 6''-O-acetyl isoquercitrin 1.0-6.0 %, quercitrin 0.5-4.0 %, trifolitin 0.2-3.0 %, etc. The kinder leaf total flavone extract is applied in preparing medicine for treating cardiac and cerebral ischemia.
Owner:周亚球 +1
Isoquercitrin clathrate and preparation thereof
InactiveCN101301477ASimple manufacturing processHigh inclusion rateOrganic active ingredientsPharmaceutical non-active ingredientsFood additiveSolubility
The present invention provides a clathrate compound formed by isoquercitrin and [beta]-cyclodextrin or derivatives thereof. The weight ratio of the isoquecitrin and the [beta]-cyclodextrin or the derivatives thereof is 1:2 to 20. The preparation method comprises the following steps of: dissolving the [beta]- cyclodextrin or the derivatives thereof into distilled water; putting the isoquercitrin into an organic dissolvant to dissolve; adding the isoquercitrin slowly into the water solution of the [beta]- cyclodextrin or the derivatives thereof, controlling the temperature and stirring; keeping stand, pumping-filtrating or directly condensing, vacuum drying, and obtaining the isoquercitrin clathrate compound. The solubility of the obtained isoquercitrin clathrate compound is significantly improved, and the isoquercitrin clathrate compound can be further developed into multiple solid dosage forms or liquid dosage forms which are suitable for medicines or food additives.
Owner:SHANXI UNIV +1
Hypericum perforatum L. total flavone extracts, its preparation and application
InactiveCN1803787ASignificant anti-hepatitis BSignificant anti-ischemic effectOrganic active ingredientsPowder deliveryHypericum perforatumAdditive ingredient
Disclosed is a Hypericum perforatum flavones extract, its preparation and use. the content of the flavones in the extract is 80-95%, the flavone-containing ingredients include birutan 5.0-50.0%, hyperoside 6.0-70.0%, isoquercitrin 3.0-10.0%, quercetin-3-O-alpha-L-arabinoside 0.5-5.0%, quercetin 0.5-5.0%, meletin 0.1-1.0%, and other balancing flavones components.
Owner:周亚球 +1
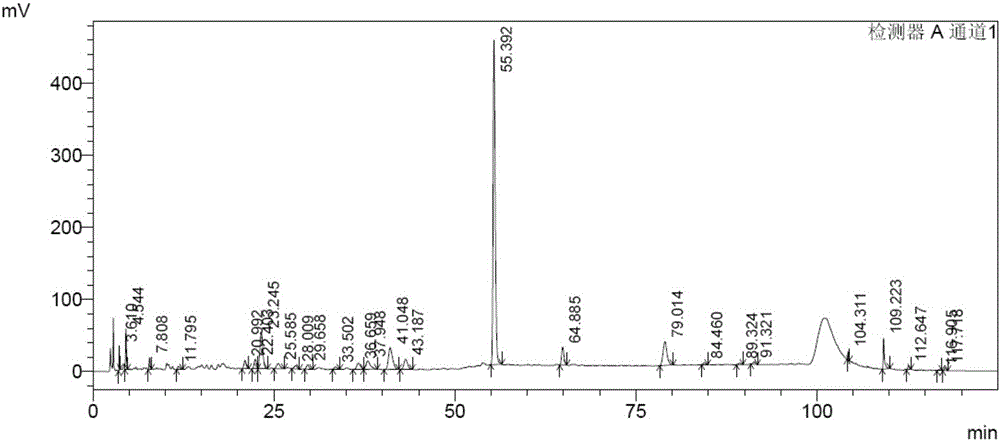
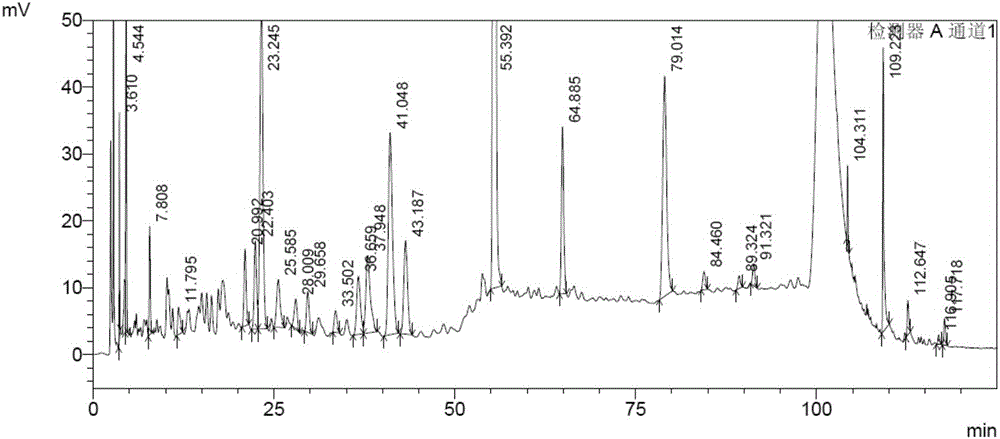
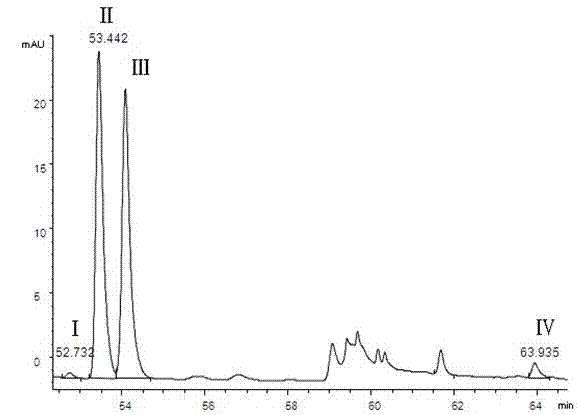
Bushy knotweed leaf fingerprint HPLC method and its use in bushy knotweed leaf capsule quality control
ActiveCN106018629AHigh separation of chromatographic peaksSolving Quality Control IssuesComponent separationGoniothalamusHplc method
The invention provides a bushy knotweed leaf fingerprint HPLC method. The method comprises preparation of a sample to be detected and HPLC detection of the sample. The method can simultaneously detect 12 index components such as gallic acid, chlorogenic acid, ferulic acid, polydatin, rutin, isoquercitrin, quercitrin, resveratrol, quercetin, rheum emodin, chrysophanol and physcion in bushy knotweed leaf raw drugs and their products. The bushy knotweed leaf fingerprint HPLC method has a chromatographic peak resolution, realizes retention time stabilization of different samples and solves the bushy knotweed leaf capsule quality control problems.
Owner:云南海沣药业有限公司
Method for separating rutin, hyperoside, isoquercitrin and quercetin from lotus leaves
InactiveCN102827220AImprove separation efficiencySugar derivativesSugar derivatives preparationStationary phaseGradient elution
The invention relates to the technical field of traditional Chinese medicine, especially to a method for separating rutin, hyperoside, isoquercitrin and quercetin from lotus leaves. The method for separating the rutin, the hyperoside, the isoquercitrin and the quercetin from the lotus leaf comprises separating components in the lotus leaves powder by using a column chromatography method for a gradient elution for 65 to 75 minutes with a flow rate of 0.6 to 0.8 ml / min under a temperature of 28 to 32 DEG C and a pH value of 3.5 to 4.5, wherein a stationary phase of the column chromatography is C18, a mobile phase A is a phosphatic buffer with a pH value of 3.5 to 4.5, a mobile phase B is acetonitrile, and the outflow components are successively the rutin, the hyperoside, the isoquercitrin and the quercetin according to a time sequence; respectively recovering eluents containing the rutin, the hyperoside, the isoquercitrin and the quercetin; and finally removing solvents with reduced pressure distillation to obtain the rutin, the hyperoside, the isoquercitrin and the quercetin. The method not only can separate the rutin, the hyperoside, the isoquercitrin and the quercetin, but also is high is separation efficiency.
Owner:湖州市食品药品检验所
Method for separating and purifying hyperoside and isoquercitroside from aurea helianthus
ActiveCN107759648AReduce consumptionSimplified processing stepsSugar derivativesOrganic chemistry methodsHyperosideZinc salts
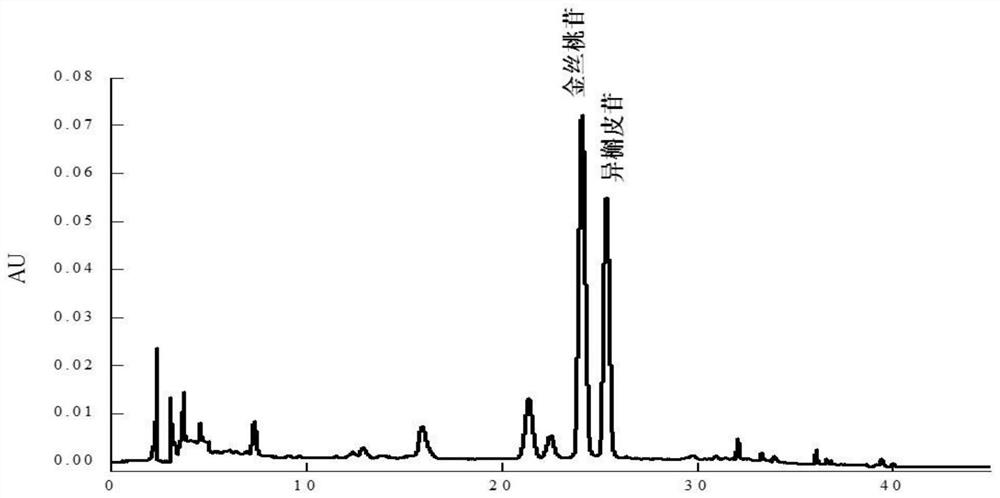
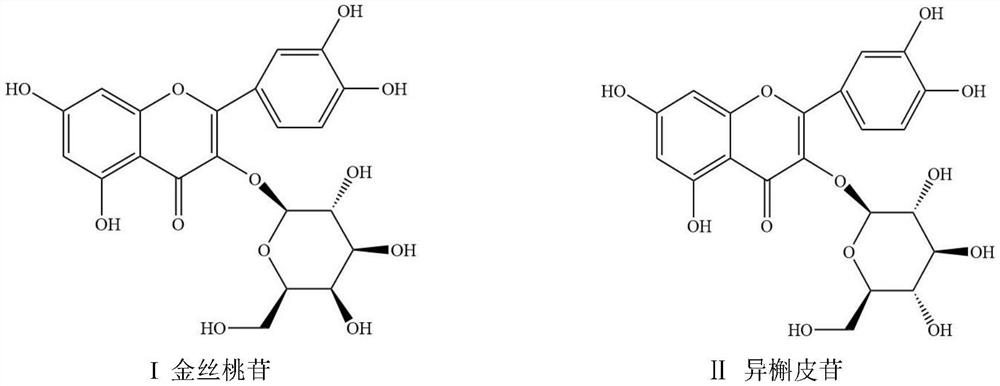
The invention relates to a method for separating and purifying hyperoside and isoquercitroside from aurea helianthus, belonging to the field of extraction and separation of natural compounds. The method comprises the following steps: heating, refluxing, and extracting flavones in the aurea helianthus; under alkaline condition, forming stable complex precipitate by zinc salt with flavonoids exceptthe hyperoside and isoquercitroside in the aurea helianthus; centrifuging to remove the complex precipitate; and concentrating supernatant, thus obtaining high-purity hyperoside and isoquercitroside.The method provided by the invention can be used for rapidly, efficiently and safely separating and purifying the hyperoside and isoquercitroside from the aurea helianthus and is beneficial to realization of industrial production of the hyperoside and isoquercitroside.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Total giamt St.John' swort Herb flavone extract, its preparing method and use
InactiveCN1821255ASignificant anti-ischemic effectGood curative effectPowder deliverySugar derivativesVascular diseaseTraditional medicine
The present invention features that the extract has total flavone content of 50-90 wt%, and the total flavone consists of rutin 5.0-15.0 wt%, hyperoside 8.0-25.0 wt%, isoquercitrin 10.0-30.0 wt%, quercetin 2.0-7.0 wt% and kaempferol swartziol 0.8-2.5 wt% except other flavone components. The extract may be used in preparing medicine for treating cardiac and cerebral vascular diseases.
Owner:周亚球 +1
Edible compositions containing stabilized natural colorants
Owner:WM WRIGLEY JR CO
Method for determining content of chemical components in chrysanthemum
ActiveCN111537653AHigh sensitivityFast analysisComponent separationMedicinal herbsIsochlorogenic acid
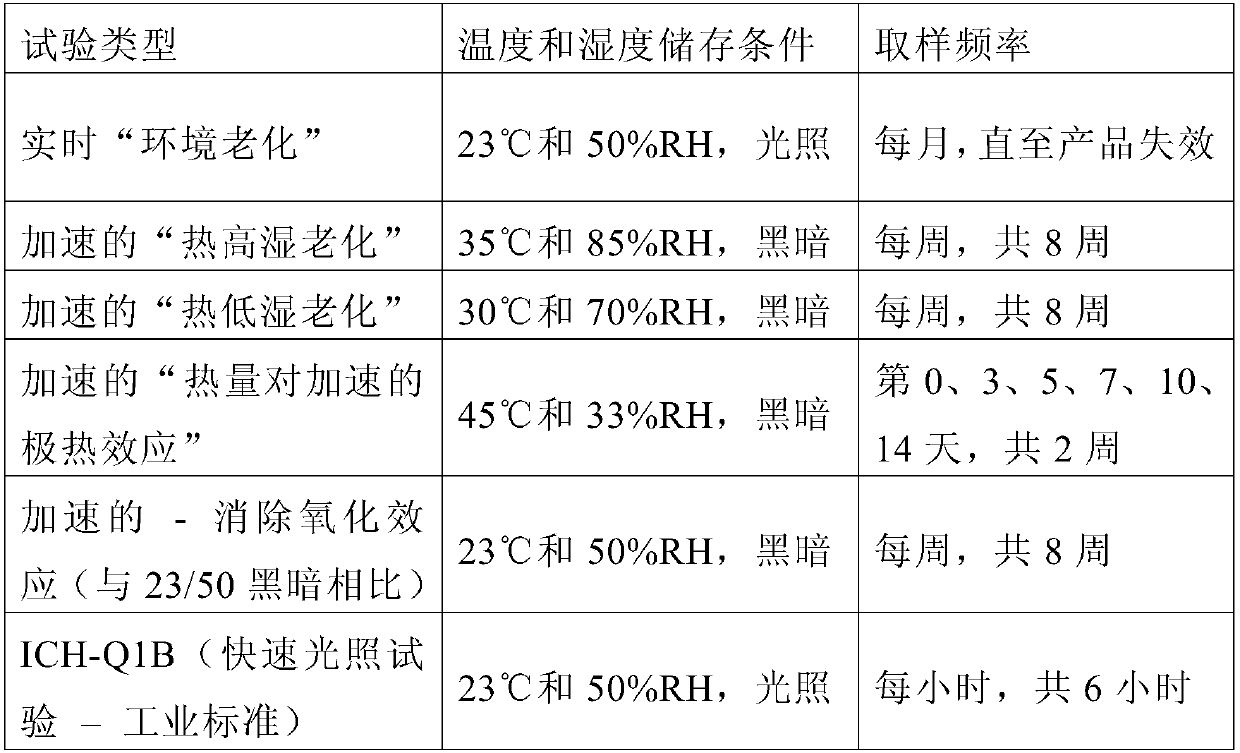
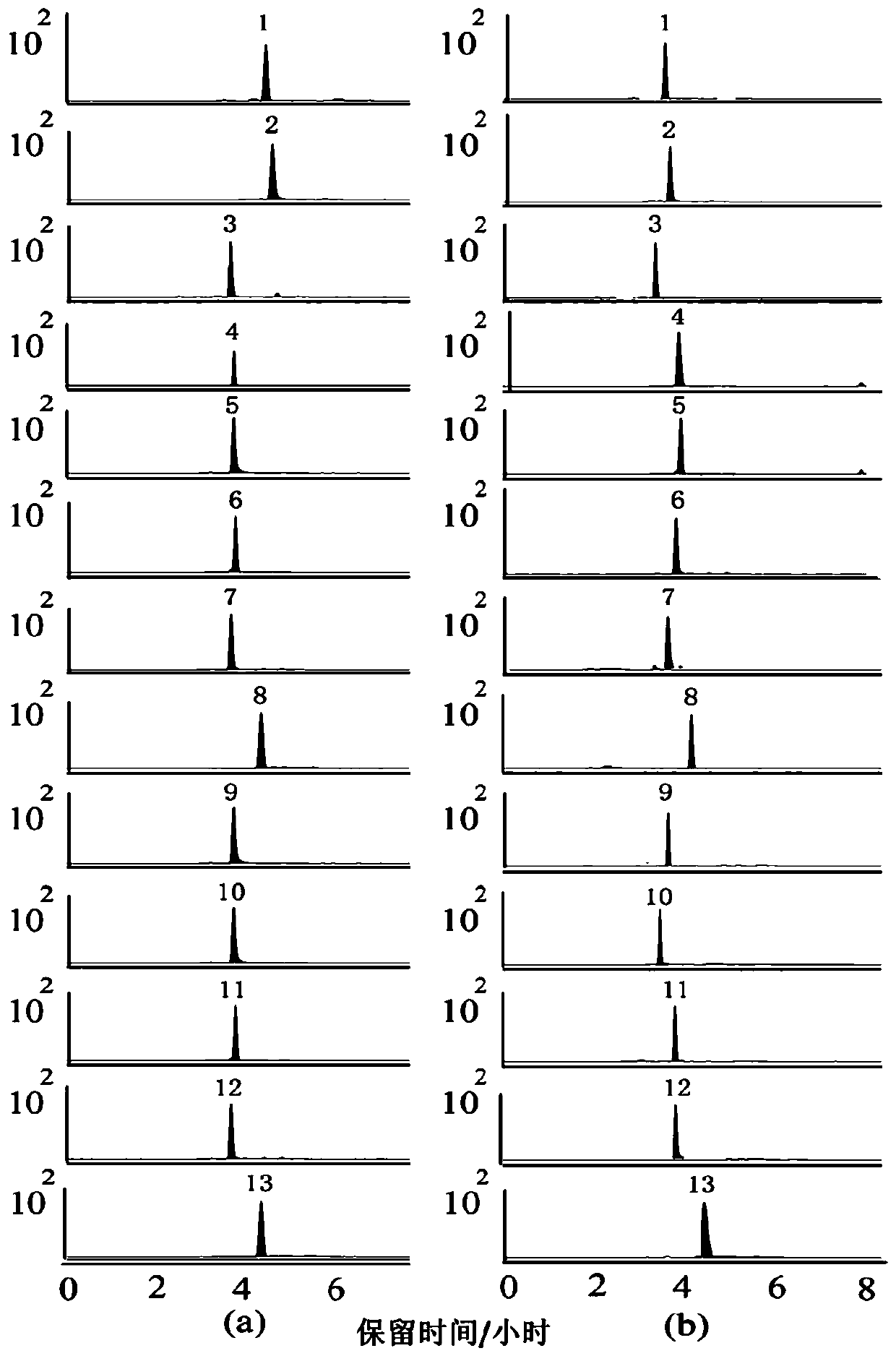
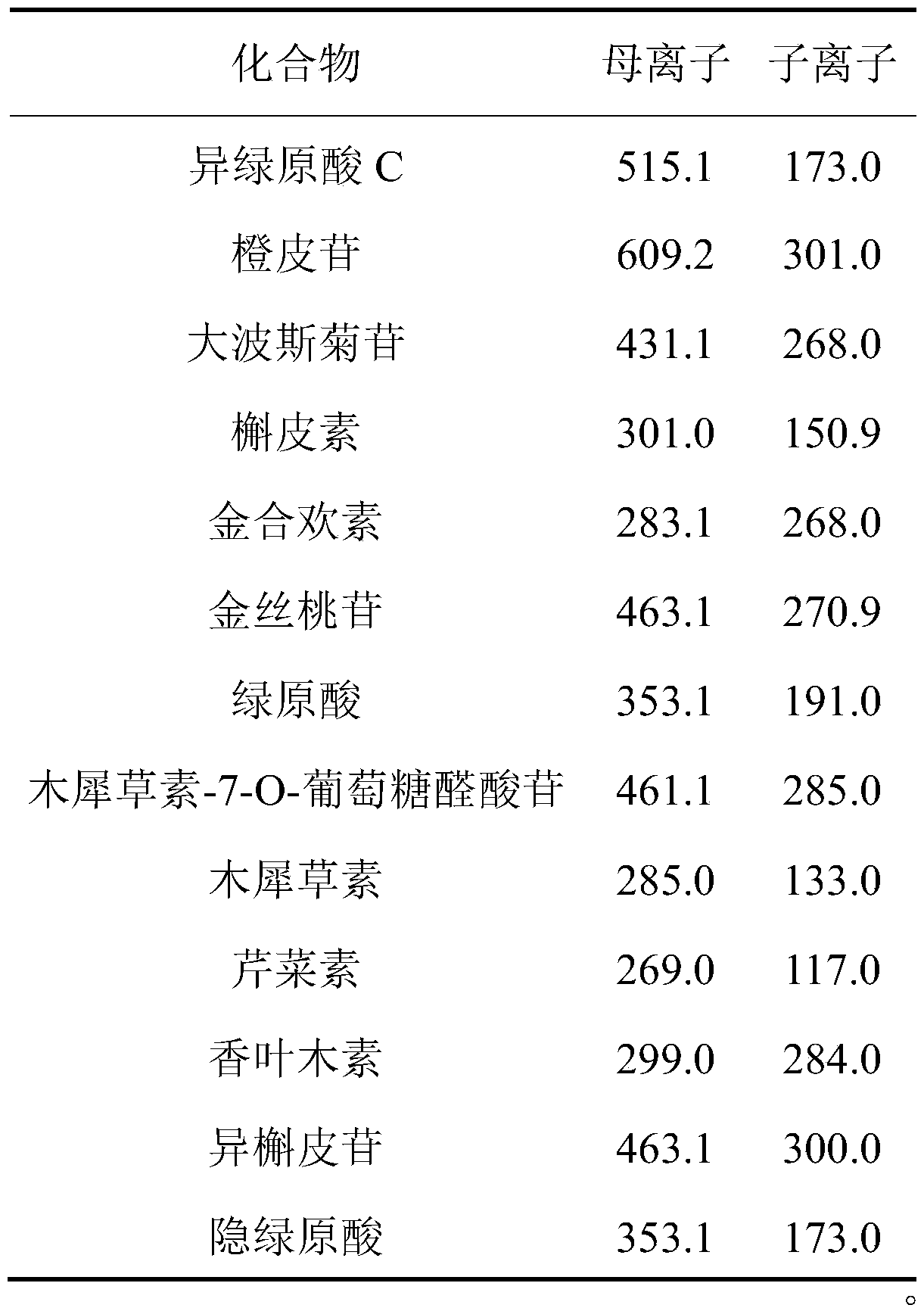
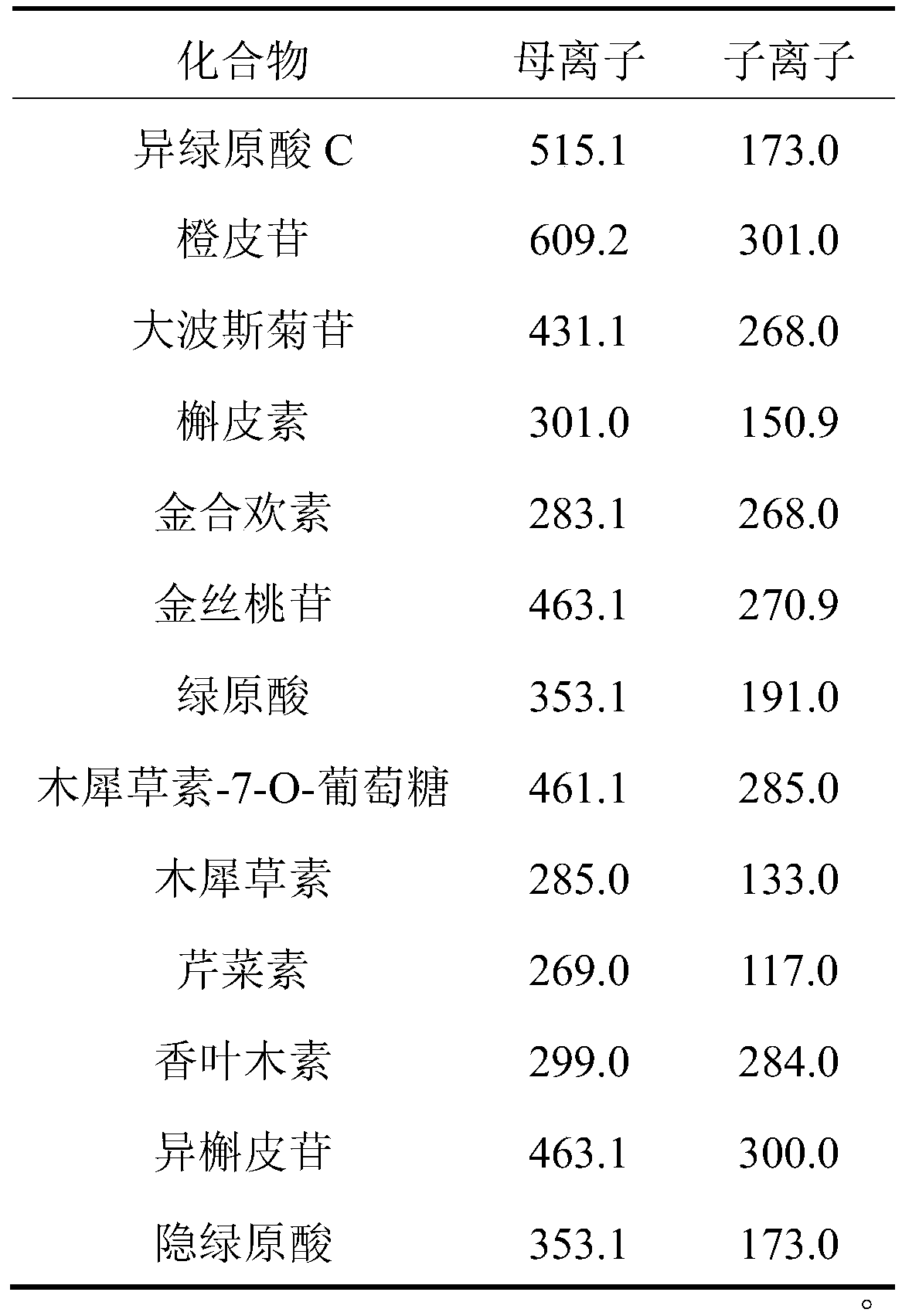
The embodiment of the invention provides a method for determining the content of chemical components in chrysanthemum, which adopts ultra-high performance liquid chromatography-mass spectrometry to determine the content of 13 chemical components in chrysanthemum at the same time. The 13 chemical components comprise isochlorogenic acid C, hesperidin, coreopsis tinctoria glycoside, quercetin, farnetin, hyperoside, chlorogenic acid, luteolin-7-O-glucuronide, unuronide, luteolin, apigenin, diosmetin, isoquercitrin and cryptochlorogenic acid. By adopting the method disclosed by the invention, the contents of 13 chemical components in the chrysanthemum can be simultaneously determined by reasonably selecting chromatographic conditions and mass spectrometric conditions. And the method has the advantages of simplicity, convenience, high sensitivity, high analysis speed, strong specificity and the like, so that the method can be used for controlling the quality of the chrysanthemum medicinal material.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Application of isoquercitrin as inhibitor of calcium ion channel
The invention relates to application of isoquercitrin as an inhibitor of a calcium ion channel, and belongs to the field of medicine. The invention also provides application of the isoquercitrin in preparation of drugs for treating cardiovascular diseases, coronary heart diseases, atherosclerosis, advanced renal failure, neurological diseases, chronic pain, acute pain or inflammatory diseases. Theinvention proves that the isoquercitrin can inhibit the calcium ion channel, especially a TRPC ion channel, and expands the application of the isoquercitrin.
Owner:JIANGSU SUZHONG PHARM GRP CO LTD +1
Compositions that protect cells from oxidative and mitochondrial stress
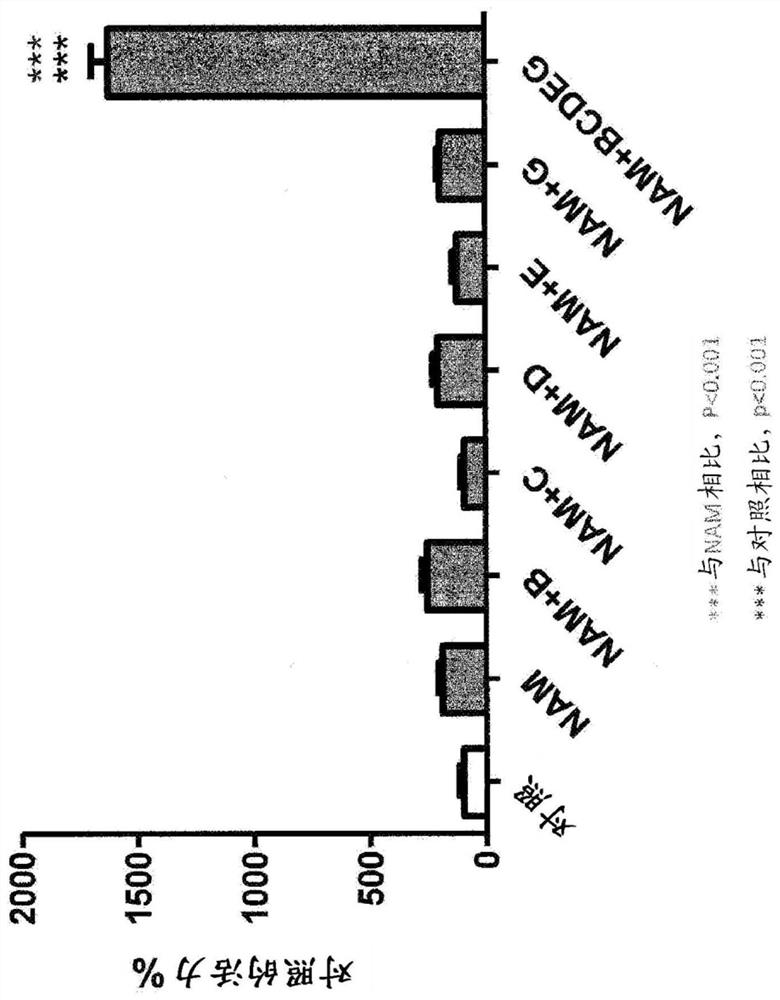
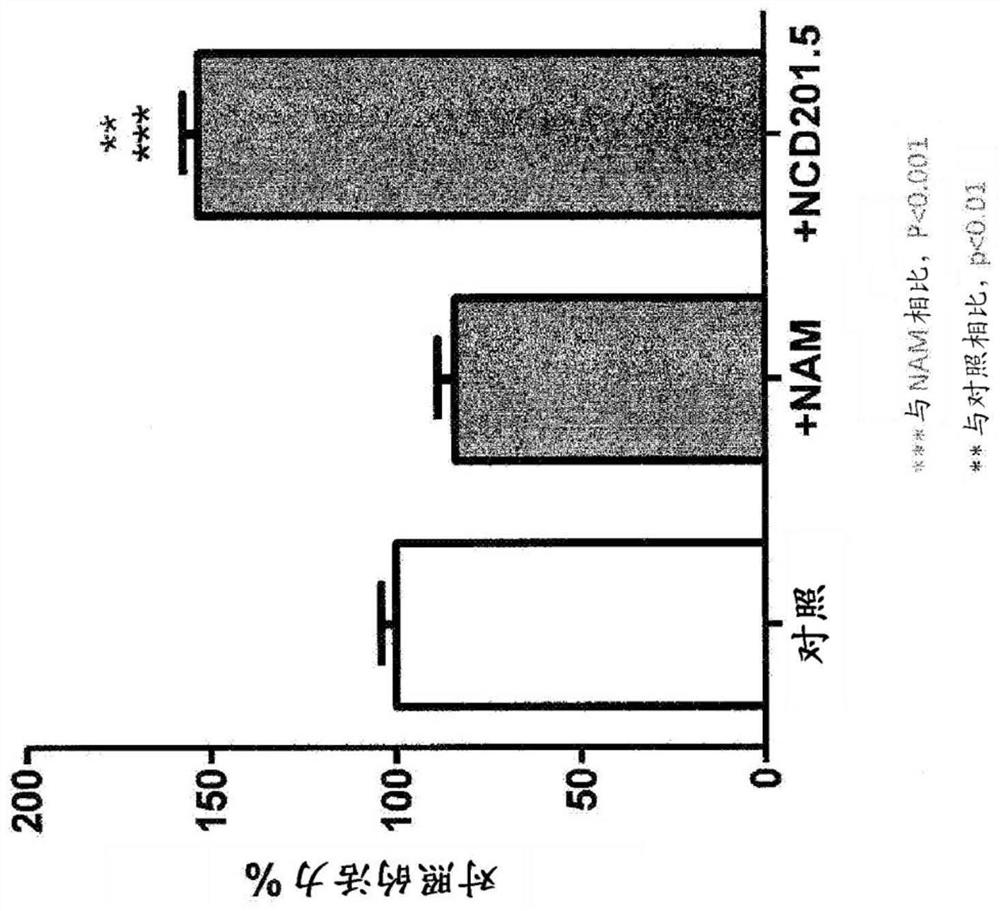
There are described compositions comprising an effective amount of a combination of two or more components, said components selected from acacetin, ACT1 peptide, alpha-lipolic acid, alprostadil, anisomycin, apigenin, ascorbic acid, astragalus, berberine, beta-lapachone, beta-hydroxy-beta-methyl-butyrate, bacopa monnieri, catechin, catechol, chamomile, chrysin, coumestrol, curcumin, dinitrophenol,dinoprost, ellagic acid, (-)-epigallocatechin gallate, green tea extract, fisetin, genistein, ginsenoside RE, glabridin, 18-alfa-glycyrrhetinic acid, 18-beta-glycyrrhetinic acid, glycyrrhizin, hydroquinone, isoquercitrin (EMIQ), kaempferol, kuromanin, leucine, lithium, luteolin, luteolin, luteolinidin, melatonin, menadione, 1-methylnicotinamide (MNA), methyl salicylate, myricetin, nadide, niacin (vitamin B3), nicotinamide (NAM), nicotinamide mononucleotide (NMN), nicotinamide riboside (NR), nicotinic acid adenine dinucleotide (Na AD), nicotinic acid mononucleotide (Na MN), parsley (petroselinium crispum), phenylephrine, pokeweed mitogen, 15-Delta prostaglandin J2, puromycin, quercetin, quinolinic acid, retinoic acid, trichostatin A, troxrutin, rutin, tryptophan, vitamin D3, withaferin A, wortmannin and zinc (including salts thereof).
Owner:努其杜有限公司
Chan tablet liquid chromatography-mass spectrometry determination method
PendingCN114113403AComponent separationAgainst vector-borne diseasesSalvianolic acid BO-Phosphoric Acid
The invention relates to a liquid chromatography-mass spectrometry determination method for a Holdan tablet. The method comprises the following steps: mixing psoralen glycoside, isopsoralen glycoside, hyperoside, quercetin 3-O-glucuronide, isoquercitrin, sennoside B, astragalus smicus glycoside, sennoside A, rosmarinic acid, alkannic acid, salvianolic acid B, nuciferine, salvianolic acid A, psoralen, isopsoralen, salvianolic acid C, rhein, neopsoralen isoflavone, psoralen and cryptotanshinone, and uniformly stirring to obtain a mixture; the method comprises the following steps of: preparing reference substance solutions A and B and a test sample solution by taking a sample as a reference substance and bakuchiol as a reference substance, injecting the reference substance solutions A and B and the test sample solution into a liquid chromatograph-mass spectrometer, obtaining mass spectrograms when A is in a positive ion mode and B is in a negative ion mode, and calculating the content of the effective components in the test sample according to the mass spectrograms under the chromatographic conditions that an Agilent C18 chromatographic column (4.6 mm * 250 mm, 5 [mu] m), the flow rate is 0.4 mL.min <-1 > and the sample injection volume is 2 [mu] L. The column temperature is 30 DEG C, and a 0.2% phosphoric acid aqueous solution (A)-acetonitrile (B) is used as a mobile phase for gradient elution.
Owner:NANCHANG JISHUN PHARMA CO LTD
Water lily flower extract as well as preparation method and application thereof
ActiveCN114469814AQuality improvementImprove qualityCosmetic preparationsToilet preparationsBiotechnologyOrganosolv
The invention discloses a water lily flower extract as well as a preparation method and application thereof, and belongs to the technical field of plant extraction. The preparation method comprises the following steps: (1) adding a dried nymphaea tetragona flower product into an organic solvent aqueous solution with the volume ratio of 50%-90% according to the solid-liquid ratio of 1g: 20mL-60mL, extracting at the temperature of 20-65 DEG C, and separating to obtain an extracting solution; and (2) adjusting the pH value of the extract to 7-9, then removing the organic solvent, and drying to obtain the nymphaea candida presl extract. By means of the technology, efficient enrichment and extraction of skincare functional components in the nymphaea candida presl are achieved, and the content of main components ellagic acid and isoquercitrin in the extract is relatively high. The extract has strong oxidation resistance and wrinkle resistance, and has great application prospects in the fields of cosmetics, food, medical products and the like.
Owner:ZHEJIANG UNIV +1
Multi-component content detection of ginkgo armillaria mellea oral solution and construction of a fingerprint spectrum method of the ginkgo armillaria mellea oral solution
The invention relates to multi-component content detection of a ginkgo armillaria mellea oral solution and construction of a fingerprint spectrum method of the ginkgo armillaria mellea oral solution. The content detection analysis method established by the invention is good in precision, reproducibility, stability and sample recovery rate. Therefore, the content detection method is simple and convenient to operate, accurate and reliable, and can be used for determining the content of the gingko armillaria mellea oral solution. According to the fingerprint spectrum detection method, 19 characteristic fingerprint peaks in the gingko armillaria mellea oral solution can be detected at the same time, and 15 fingerprint peaks, namely uridine, adenosine, guanosine, acesulfame potassium, 5-hydroxymethylfurfural, protocatechuic acid, p-hydroxybenzoic acid, sodium benzoate, rutin, isoquercitrin and the like, are identified through a liquid chromatography-mass spectrometry technology and comparison of a reference substance. The detection method can be used for controlling the quality of the gingko armillaria mellea oral solution.
Owner:QIONGLAI TIANYIN PHARM CO LTD
Detection method of gingko flavonol glycosides
ActiveCN112697899AAccurately reflectEffective reflectionComponent separationGlycosideDrugs preparations
The invention discloses a detection method of gingko flavonol glycosides. The detection method comprises the following steps of: (1) preparing reference substances and a test solution, the reference substances being rutin, hyperoside, isoquercitrin, quercetin-3-O-2''-glucose rhamnoside, kaempferol-3-O-beta-D-rutinoside, narcissoside, syringetin-3-O-beta-D-rutinoside, cosmosiin, quercetin-3-O-p-coumaroyl rhamnose glucoside and kaempferol-3-O-p-coumaroyl rhamnose glucoside reference substances; (2) performing detection; (3) establishing a standard fingerprint spectrum; and (4) calculating the content of common peaks in the fingerprint spectrum established in the step (3). According to the detection method, a specific standard fingerprint spectrum of the ginkgo flavonol glycosides is established, the content of main flavonol glycosides in gingko and related extracts and pharmaceutical preparations thereof can be accurately detected, and the accuracy, precision, reproducibility and stability are good.
Owner:CHINA PHARM UNIV
Method for preparing hyperin from Dogbane leaves
InactiveCN102190693BHigh purityReduce consumptionSugar derivativesSugar derivatives preparationApocynum venetumFlavonoid glycosides
The invention discloses a method for preparing hyperin from Dogbane leaves, which comprises the following steps: firstly. determining the optimum extraction temperature, a separation material and the concentration of an eluting agent; secondly, regulating the pH value, and extracting with a solvent having high hyperin dissolving and extracting power, thus obtaining a liquid extract; thirdly, heating at 80-85 DEG C under reflux of ethanol or methanol, and extracting; and finally, standing and crystallizing at a preferable cooling temperature, thus effectively separating the hyperin and isoquercitrin which are two flavonoid glycoside components having similar properties. In the invention, the hyperin is further purified by preparing a liquid phase; and a moving phase is examined and determined by methodology, and is subjected to targeted and efficient separation and purification based on the absorption wavelength of the hyperin, thus obtaining the hyperin having purity up to 90-99%. Thehyperin conforms to the requirements for Class A active pharmaceutical ingredients.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
Application of alpha-L-rhamnoside enzyme in directional synthesis of isoquercitrin by biological conversion of rutin
ActiveCN101985639BImprove catalytic conversionLow costOn/in organic carrierFermentationPtru catalystEnzyme catalysis
The invention relates to application of an alpha-L-rhamnoside enzyme in the directional synthesis of isoquercitrin by the biological conversion of rutin. Because the crude enzyme preparation or the immobilized enzyme preparation of the alpha-L-rhamnoside enzyme is used for catalyzing and hydrolyzing the rutin and directionally and biologically synthesizing the isoquercitrin, the application has the advantages of wide source, easy preparation and low cost of catalysts, high stability, and high catalysis efficiency and specificity of the enzyme preparation, and is easy to store. Thus, the alpha-L-rhamnoside enzyme can greatly reduce the production cost of quercetin, the catalysis and conversion rate of the enzymes is high, and the products are single.
Owner:JIANGSU UNIV OF SCI & TECH
Chishui dendrobium nobile flower component content determination method
ActiveCN114034797AHigh sensitivityMeet the requirements of method validationComponent separationContent determinationRhamnose
The invention discloses a Chishui dendrobium nobile flower component content determination method which comprises the following steps: S1, preparation of a reference substance solution: respectively precisely weighing liquiritin, kaempferol-3-glucose rhamnoside and an isoquercitrin standard substance, and dissolving in a solvent to obtain a mixed reference substance solution; s2, preparing a Chishui dendrobium nobile flower test solution; and S3, content determination: determining by adopting a high performance liquid chromatography. According to the determination method, the contents of three chemical components including liquiritin, kaempferol-3-glucose rhamnoside and isoquercitrin in the dendrobium nobile lindl flowers are determined, and the quality of the Chishui dendrobium nobile lindl flowers can be better controlled.
Owner:ZUNYI MEDICAL UNIVERSITY
Content determination method for Sedum aizoon medicinal material and product thereof
PendingCN114034801ADetection contentQuality assuranceComponent separationAgainst vector-borne diseasesMedicinal herbsGallic acid ester
The invention discloses a method for determining the content of a Sedum aizoon medicinal material and a product thereof. The invention establishes a method for simultaneously determining the content of seven chemical components, namely gallic acid, myricetin, isoquercitrin, guaijaverin, quercitrin, myricetin-3-O-(2 '-gallyl)-alpha-L-rhamnopyranoside and quercetin-3-O-(2'-gallyl)-rhamnoside, in the Miao medicine Sedum aizoon. The method can be used for simultaneously determining seven chemical components in the Sedum aizoon, has the advantages of high determination efficiency, favorable precision, favorable reproducibility and favorable linear relationship, is stable and reliable, efficiently detects the content of effective components in June Reyang, and effectively ensures the product quality.
Owner:贵州中医药大学
Method for manufacturingα-glycosylisoquercitrin, intermediate product and by-product thereof
The present invention provides a method for producing isoquercitrin, α-glycosylisoquercitrin, and rhamnose, the method comprising a step of naringin-degrading enzyme treatment during the isoquercitrin production from rutin in the presence of an edible component, such as gelatin, wheat gluten, chitosan, lecithin, a glycerol fatty acid ester, xanthan gum, carrageenan, sodium chondroitin sulfate, casein, enzymatically decomposed gelatin, sodium alginate, konjac extract, gellan gum, guar gum, soybean protein, agar, pectin, yeast extract, egg-white peptide, cluster dextrin, gum arabic, arginine, sodium metaphosphate, karaya gum, locust bean gum, sodium pyrophosphate, glucosamine, chitin, sodium glutamate, dextrin, trehalose, or a grain-based food ingredients. According to the present invention, isoquercitrin and α-glycosylisoquercitrin, which are of use as antioxidants, anti-fading agents, flavor change inhibitors, etc., can be produced in enhanced yields.
Owner:SAN EI GEN F F I
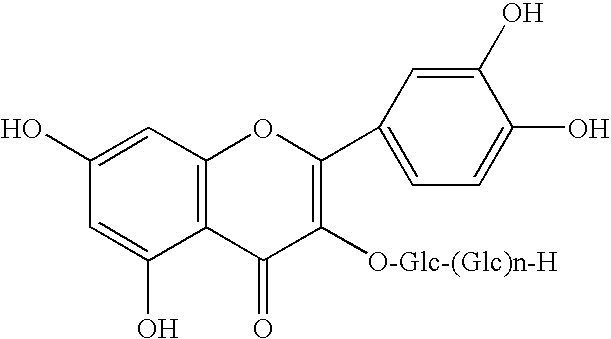
Marker for screening and identifying not-from-concentrate apple juice based on non-targeted metabolomics and use thereof
PendingUS20220187267A1Component separationMaterial analysis by electric/magnetic meansChlorogenic acidFruit juice
The present disclosure discloses a marker for screening and distinguishing NFC apple juice based on non-targeted metabolomics and use thereof, relating to the technical field of distinguishing of juice. The marker for distinguishing NFC apple juice disclosed in the present disclosure is selected from the following molecules: gallocatechin, catechin, taxifolin, p-hydroxybenzaldehyde, 5-methoxysalicylic acid, azelaic acid, caffeic acid, chlorogenic acid, epicatechin, eriodictyol, ferulic acid, isoquercitrin, naringenin, n-fructosyl isoleucine, p-coumaraldehyde, p-coumaric acid, phloretin, phlorizin, procyanidin B1, quercetin-3-O-galactoside and rutin. The above markers may be used for distinguishing the NFC apple juice and the FC apple juice, and have relatively high accuracy.
Owner:INST OF QUALITY STANDARD & TESTING TECH FOR AGRO PROD OF CAAS +1
Antibacterial agent for restraining shewanella putrefaciens, preparation method of antibacterial agent, and application of antibacterial agent to preparation of antistaling agent for south America white shrimps
PendingCN110692702AGood antibacterial effectEfficientNatural extract food ingredientsMeat/fish preservation using chemicalsBiotechnologyGallic acid ester
The invention discloses an antibacterial agent for restraining shewanella putrefaciens, and an application of the antibacterial agent to preparation of antistaling agent for south America white shrimps. The antibacterial agent is characterized in that the antibacterial agent is sedum aizoon total flavonoids, the sedum aizoon total flavonoids mainly contain quercitrin and gallic acid, and the antibacterial agent also comprises a small amount of kaempferol, a small amount of quercetin and a small amount of isoquercitrin, wherein the content of the quercitrin is 35-50% of the total weight, and the content of the gallic acid is 30-40% of the total weight. The antibacterial agent for restraining shewanella putrefaciens is applied to preparation of the antistaling agent for south America white shrimps. The antistaling agent for south America white shrimps consists of the following raw materials in parts by weight of 4 parts of the sedum aizoon total flavonoids, 2 parts of the blueberry leafpolyphenols, 3.2 parts of N-acetyl-L-cysteine and 1000 parts of water. The antibacterial agent has the advantages that blackening and corruption of the South America white shrimps can be effectively prevented and treated, and the shelf life can be prolonged.
Owner:NINGBO UNIV
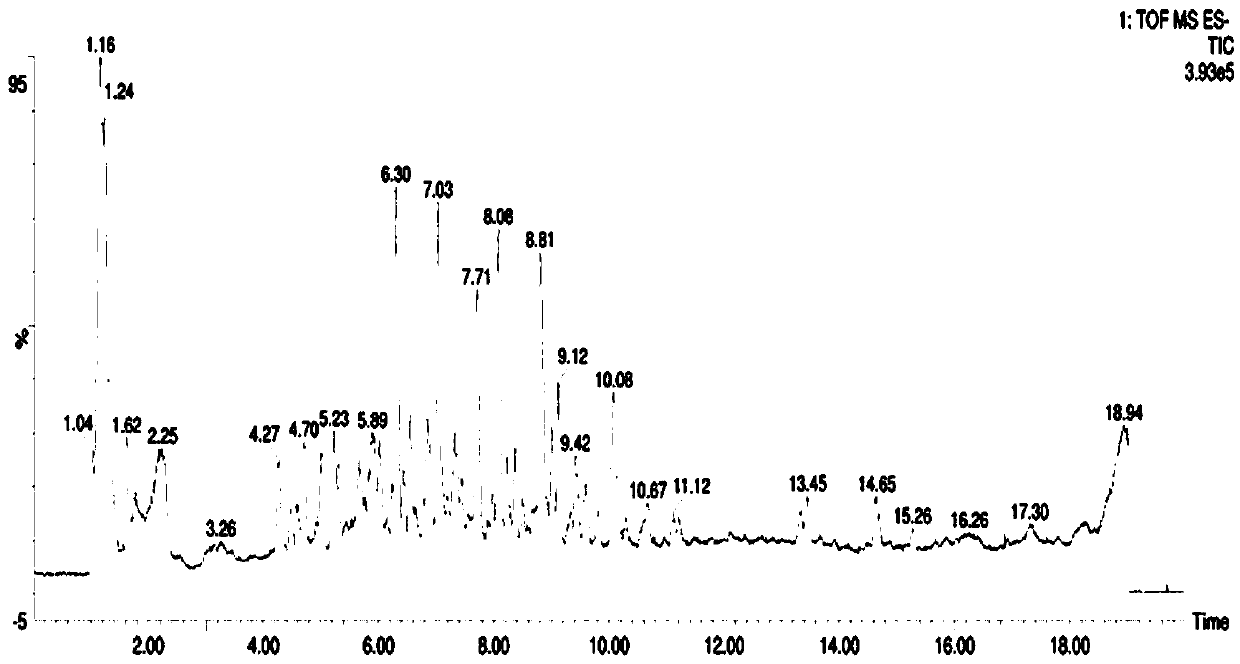
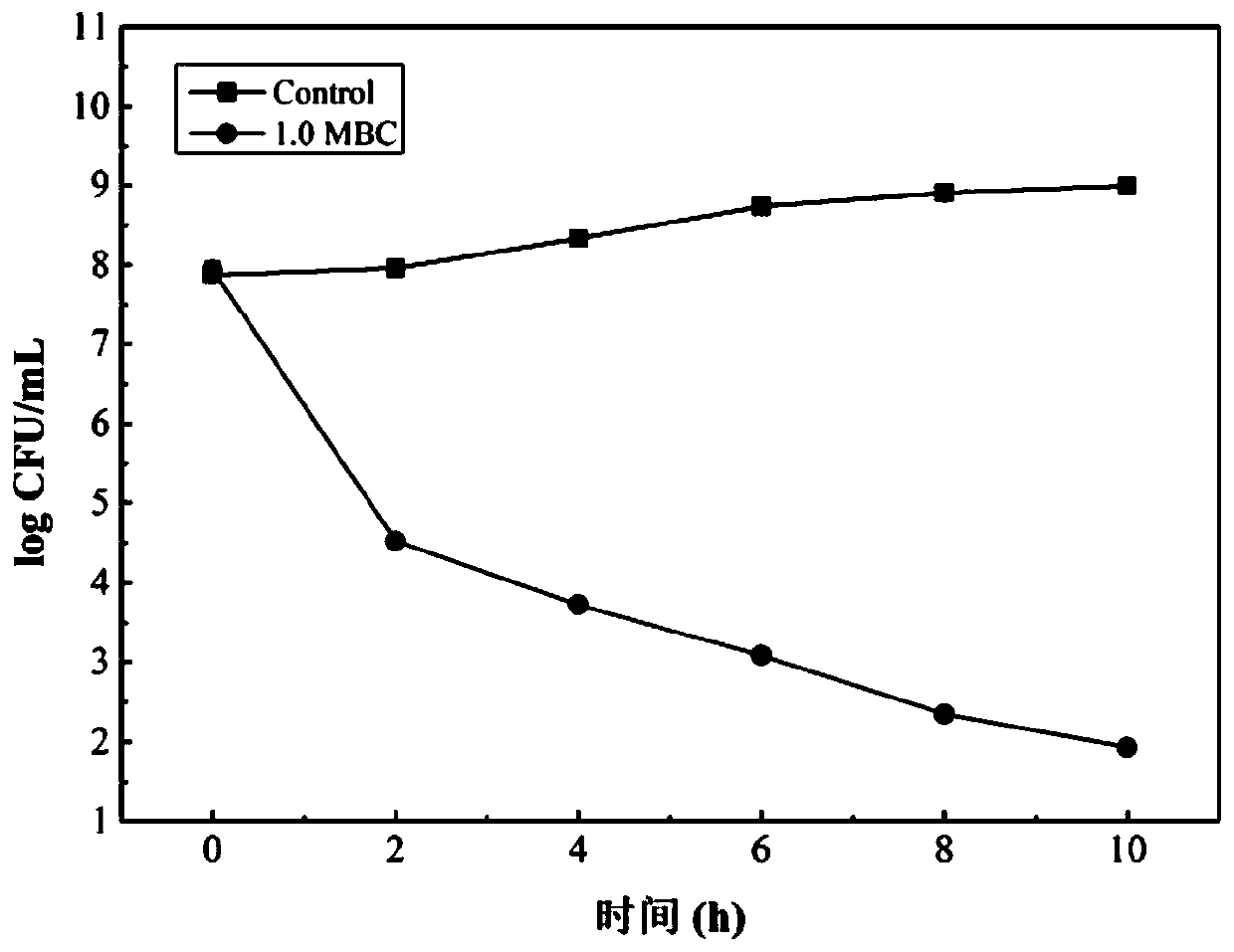
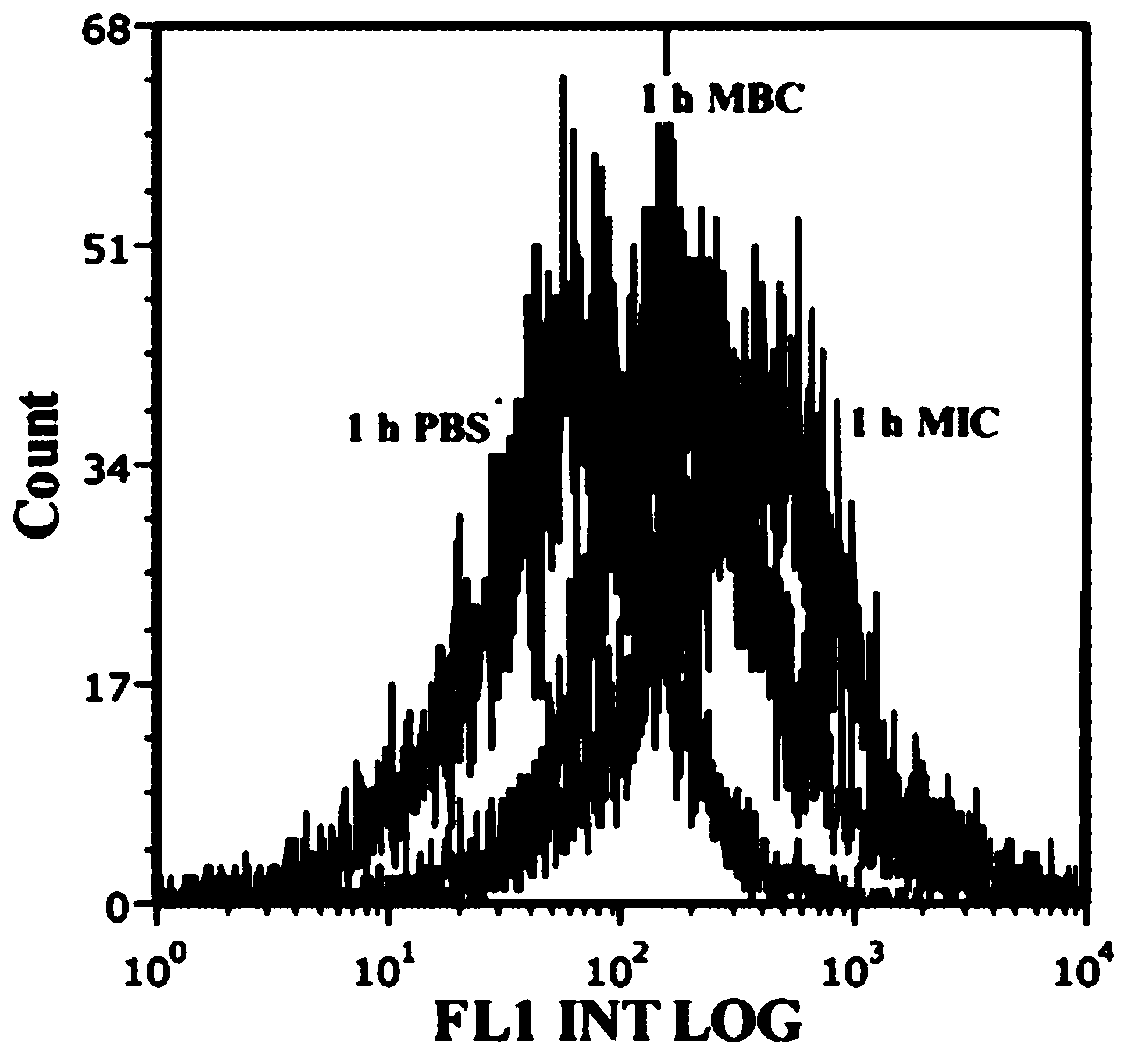
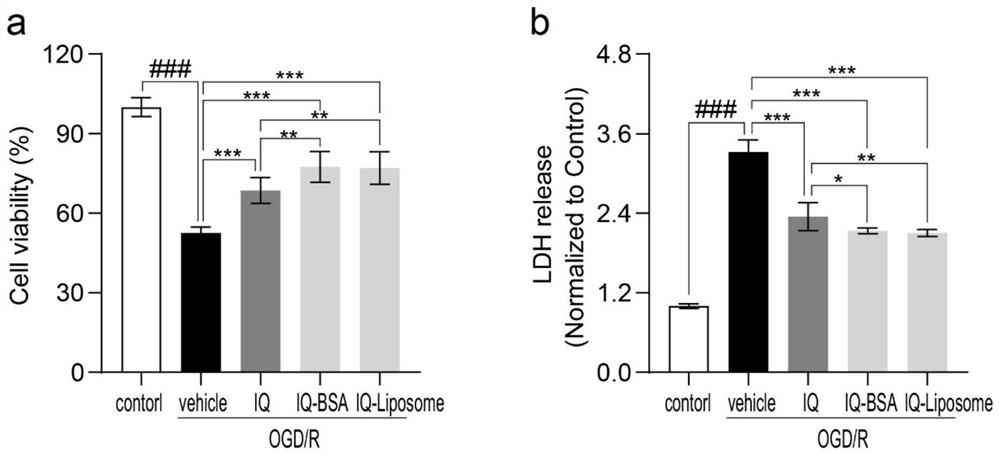
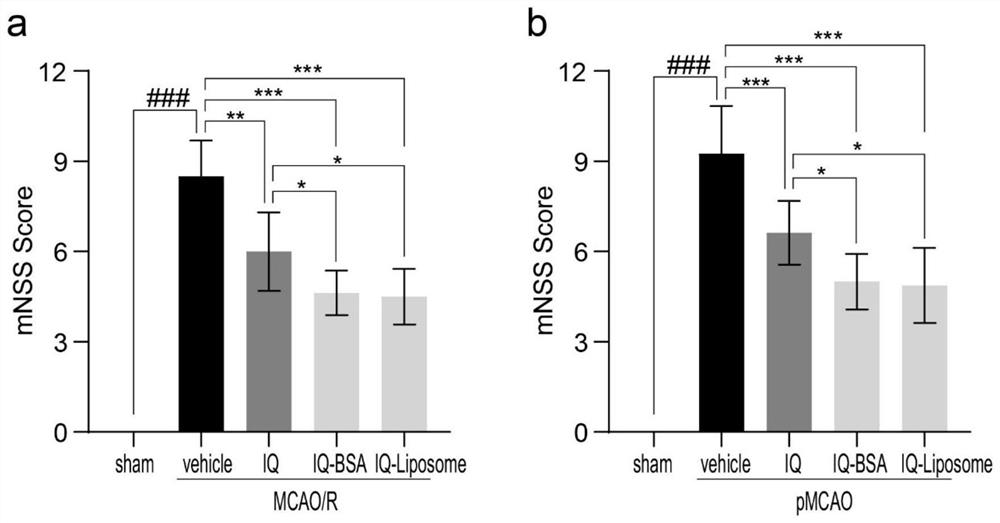
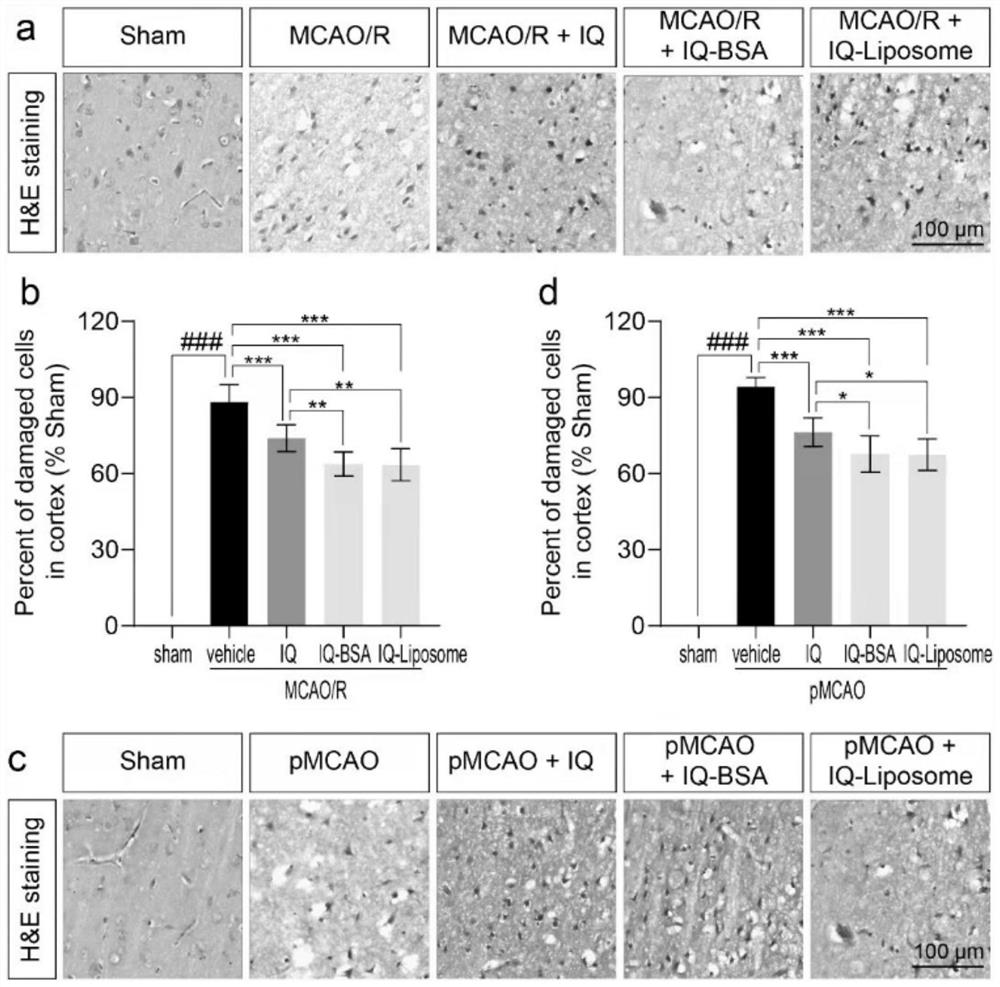
Improved isoquercitrin, preparation method and application thereof
PendingCN114010651AImprove solubilityBoth improvement and LDH release inhibition effects are significantly good solubilityOrganic active ingredientsNervous disorderDiseasePharmaceutical drug
The invention discloses improved isoquercitrin, a preparation method and application thereof. The improved isoquercitrin comprises an isoquercitrin-albumin combination body or an isoquercitrin liposome. The invention further provides a preparation method of the isoquercitrin-albumin combination body and a preparation method of the isoquercitrin lipidosome. The invention further provides application of the improved isoquercitrin. The improved isoquercitrin is applied to drugs for preventing and treating stroke and cardiovascular and cerebrovascular diseases. By changing the dosage form of the isoquercitrin, the solubility of the isoquercitrin is remarkably improved, and the in-vivo bioavailability of the isoquercitrin is improved, so that the treatment effect of the isoquercitrin on stroke and related cardiovascular and cerebrovascular diseases is remarkably improved.
Owner:NANTONG UNIVERSITY
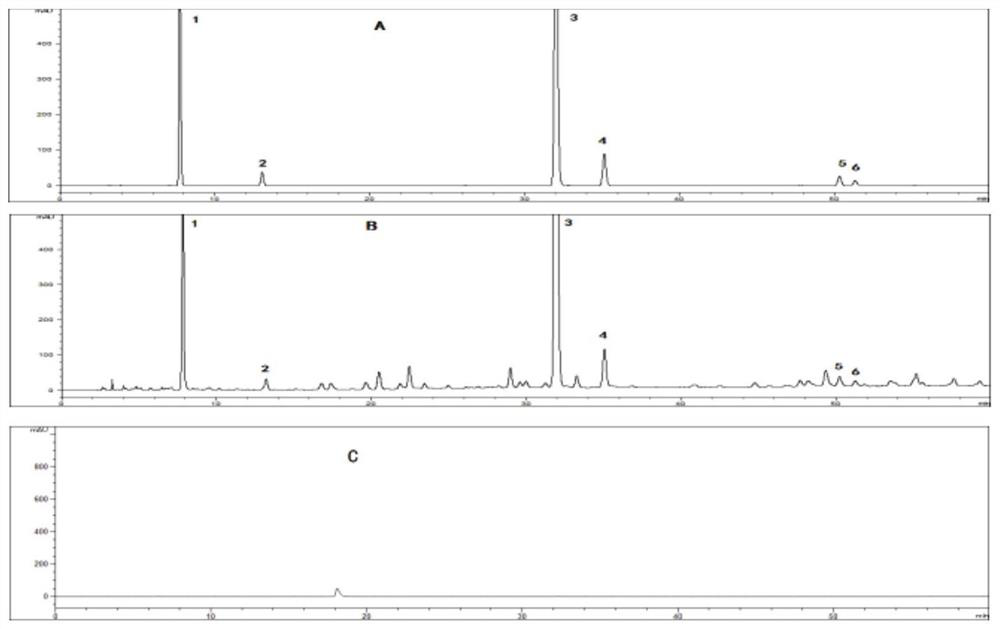
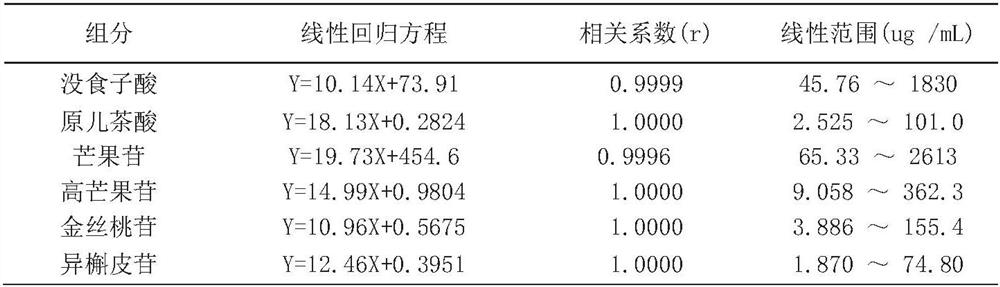
A method for determining the content of 6 components in Mango Zhike Tablets by one test and multiple evaluations by hplc method
The invention discloses an HPLC method for determining the content of 6 components of Mango Zhike Tablets by one measurement and multiple evaluations. The invention adopts the HPLC method, and the chromatographic column is a Phenomenex Gemini C18 column (4.6 mm×250 mm, 5 μm); the mobile phase is acetonitrile ( B)-0.1% phosphoric acid aqueous solution (A); gradient elution; flow rate 1.0mL / min; detection wavelength: 258nm, column temperature 30°C; one-measurement-multiple-evaluation method takes gallic acid as the internal reference, and establishes protocatechuic acid, protocatechuic acid, The relative correction factor (RCF) of mangiferin, homomangiferin, hypericin and isoquercitrin is calculated by RCF, and its measured value is compared and analyzed with the measured result of external standard method. There is no significant difference between the measurement results of the test-multi-assessment method and the external standard method (P>0.05), and the relative correction factors have good repeatability. The high-performance liquid chromatography content determination and one-test-multi-assessment method established in the present invention can be used for Mango Zhike Tablets The content determination of the six components in the method is accurate and reliable, and the repeatability is good, which can be used for the quality control of mango cough tablets.
Owner:PHARMA FACTORY OF GUANGXI TRADITIONAL CHINESE MEDICAL UNIV
Saussurea involucrata flavonoid composition
ActiveCN112870209AEnhance pharmacodynamic activityImprove absorption rateAntipyreticAnalgesicsArthritis therapySide effect
The invention relates to a saussurea involucrata flavonoid composition, which is prepared from the following four components: rutin, myristyl ether, isoquercitrin and luteolin-7-o-glucoside. A large number of animal experiments prove the pharmacodynamic activity of the flavonoid composition provided by the invention, the flavonoid composition can be used for preparing various clinically applied pain relieving and pain easing medicines and arthritis treatment medicines, and compared with a Chinese patent medicine preparation prepared by directly applying saussurea involucrata, the composition has the effects of high pharmacodynamic activity, small clinical dosage, quick response, low side effect and the like. The dosage form of the medicine can be conventional tablets or capsules, and some new dosage forms of the medicine with higher absorptivity or better storability can be prepared according to the characteristics of the medicine components.
Owner:北京慧康天诚医药科技有限公司
Method for separating rutin, hyperoside, isoquercitrin and quercetin from lotus leaves
InactiveCN102827220BImprove separation efficiencySugar derivativesSugar derivatives preparationStationary phaseGradient elution
The invention relates to the technical field of traditional Chinese medicine, especially to a method for separating rutin, hyperoside, isoquercitrin and quercetin from lotus leaves. The method for separating the rutin, the hyperoside, the isoquercitrin and the quercetin from the lotus leaf comprises separating components in the lotus leaves powder by using a column chromatography method for a gradient elution for 65 to 75 minutes with a flow rate of 0.6 to 0.8 ml / min under a temperature of 28 to 32 DEG C and a pH value of 3.5 to 4.5, wherein a stationary phase of the column chromatography is C18, a mobile phase A is a phosphatic buffer with a pH value of 3.5 to 4.5, a mobile phase B is acetonitrile, and the outflow components are successively the rutin, the hyperoside, the isoquercitrin and the quercetin according to a time sequence; respectively recovering eluents containing the rutin, the hyperoside, the isoquercitrin and the quercetin; and finally removing solvents with reduced pressure distillation to obtain the rutin, the hyperoside, the isoquercitrin and the quercetin. The method not only can separate the rutin, the hyperoside, the isoquercitrin and the quercetin, but also is high is separation efficiency.
Owner:湖州市食品药品检验所
A method for separating and purifying hyperin and isoquercitrin from goldenflower sunflower
ActiveCN107759648BReduce consumptionSimplified processing stepsSugar derivativesOrganic chemistry methodsBiotechnologyHyperoside
The invention relates to a method for separating and purifying hyperoside and isoquercitroside from aurea helianthus, belonging to the field of extraction and separation of natural compounds. The method comprises the following steps: heating, refluxing, and extracting flavones in the aurea helianthus; under alkaline condition, forming stable complex precipitate by zinc salt with flavonoids exceptthe hyperoside and isoquercitroside in the aurea helianthus; centrifuging to remove the complex precipitate; and concentrating supernatant, thus obtaining high-purity hyperoside and isoquercitroside.The method provided by the invention can be used for rapidly, efficiently and safely separating and purifying the hyperoside and isoquercitroside from the aurea helianthus and is beneficial to realization of industrial production of the hyperoside and isoquercitroside.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com