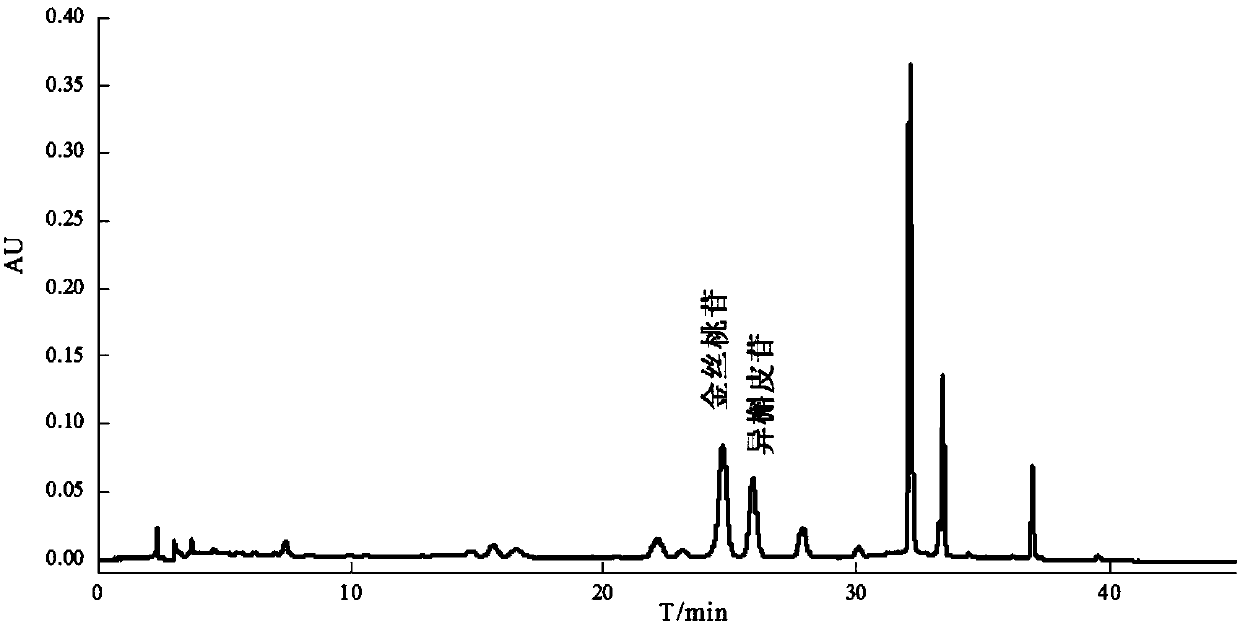
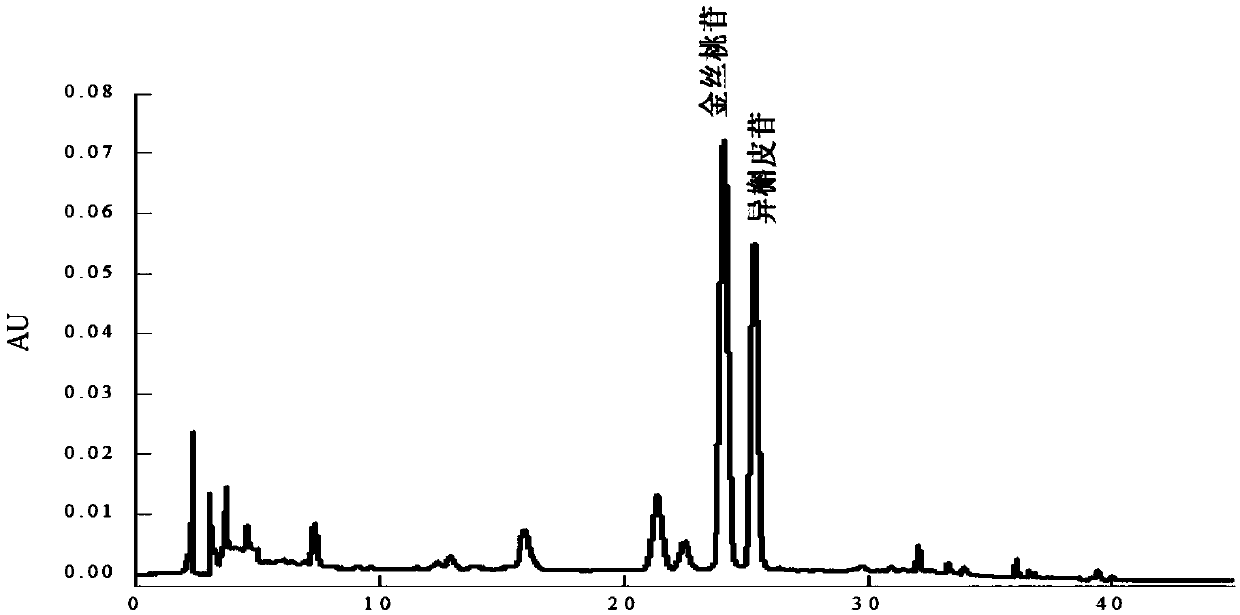
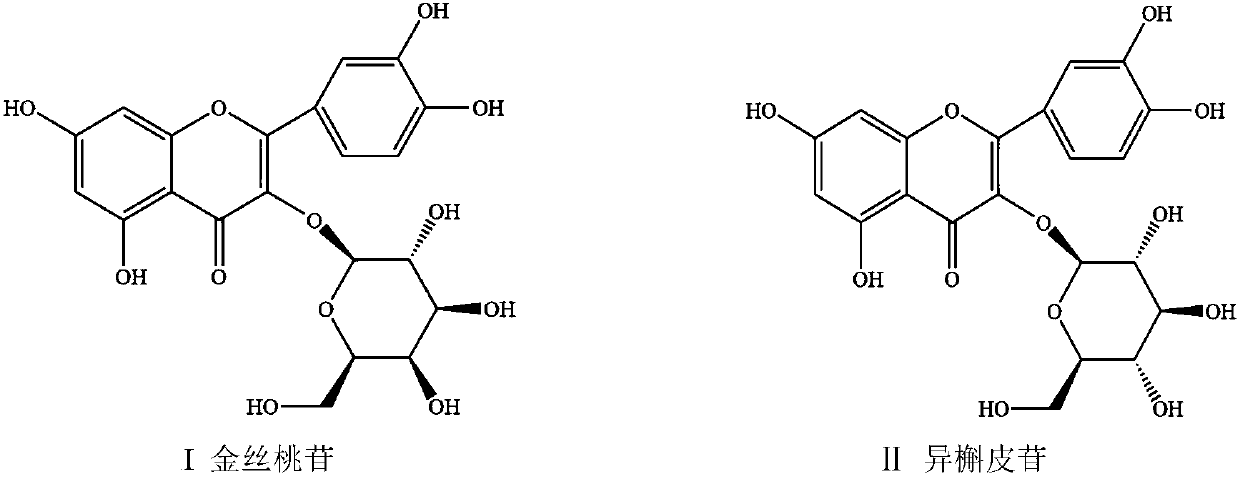
Method for separating and purifying hyperoside and isoquercitroside from aurea helianthus
A technology of hyperoside and isoquercitrin, which is applied in the field of extraction and separation of natural compounds, can solve the problems of cumbersome purification methods, difficult industrial production, and long production cycle, and achieve low cost, easy recycling, and reduced risk Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A. Accurately weigh 5.0 g of dry sunflower sunflower powder, heat and reflux extract with 70% ethanol solution for 3 hours at a temperature of 80° C., and suction filter after cooling to room temperature to obtain sunflower sunflower residue and supernatant, Collect the supernatant, and dilute it with 70% ethanol solution to a 250mL volumetric flask to obtain the sample solution;
[0023] B. Measure 10 mL of goldenflower sunflower sample solution, adjust the pH value to 10.5 with potassium hydroxide solution, add a certain mass concentration of zinc sulfate, and add an amount to ensure that the mass ratio of goldenflower sunflower sample to zinc sulfate is 1 / 1. After the addition, the complexation reaction time was 3h. The temperature of the complexation reaction is 30°C, centrifuged to obtain the supernatant and the zinc salt-flavone complex, and the supernatant is collected;
[0024] C. The above-mentioned supernatant was heated and concentrated to obtain high-purity...
Embodiment 2
[0026] A. Accurately weigh 5.0 g of dry sunflower sunflower powder, heat and reflux extract with 70% ethanol solution for 3 hours at a temperature of 80° C., and suction filter after cooling to room temperature to obtain sunflower sunflower residue and supernatant, Collect the supernatant, and dilute it with 70% ethanol solution to a 250mL volumetric flask to obtain the sample solution;
[0027] B. Measure 10 mL of goldenflower sunflower sample solution, adjust the pH value to 12.5 with sodium carbonate solution, add a certain mass concentration of zinc chloride, and add an amount to ensure that the mass ratio of goldenflower sunflower sample to zinc chloride is 1 / 0.5. After the addition, the complexation reaction time was 12h. The temperature of the complexation reaction is 40°C, centrifuged to obtain the supernatant and the zinc salt-flavone complex, and the supernatant is collected;
[0028] C. The above supernatant was heated and concentrated to obtain high-purity hyperin...
Embodiment 3
[0030] A. Accurately weigh 5.0 g of dry sunflower sunflower powder, heat and reflux extract with 70% ethanol solution for 3 hours at a temperature of 80° C., and suction filter after cooling to room temperature to obtain sunflower sunflower residue and supernatant, Collect the supernatant, and dilute it with 70% ethanol solution to a 250mL volumetric flask to obtain the sample solution;
[0031] B. Measure 10 mL of goldenflower sunflower sample solution, adjust the pH value to 11.5 with sodium hydroxide solution, add a certain mass concentration of zinc acetate, and add an amount to ensure that the mass ratio of goldenflower sunflower sample to zinc acetate is 1 / 5. After the addition, the complexation reaction time was 1 h. The temperature of the complexation reaction is 10°C, centrifuged to obtain the supernatant and the zinc salt-flavone complex, and the supernatant is collected;
[0032] C. The above supernatant was heated and concentrated to obtain high-purity hyperin and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com