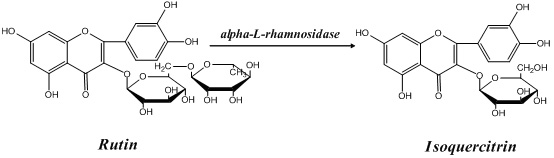
Application of alpha-L-rhamnoside enzyme in directional synthesis of isoquercitrin by biological conversion of rutin
A technology of rhamnosidase and isoquercitrin, which is applied in directions such as being fixed on/in an organic carrier, fermentation, etc., can solve the problem of failing to achieve directional hydrolysis of rutin to prepare isoquercitrin, and not yet having high selectivity. Targeted preparation of isoquercitrin and other problems, to achieve good industrial application prospects, high catalytic efficiency and specificity, and high enzymatic conversion rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Selection of strains: Select Aspergillus niger CGMCC No.2588 ( Aspergillus niger LJ-1) as a strain producing α-L-rhamnosidase;
[0032] (2) Preparation of crude enzyme solution of α-L-rhamnosidase:
[0033] Enzyme production medium formula (weight ratio): 0.1% potassium hydrogen phosphate, 0.1% potassium dihydrogen phosphate, 0.1% ammonium sulfate, 0.01% sodium nitrate, 0.01% magnesium sulfate, 0.001% ferrous sulfate heptahydrate, zinc sulfate 0.001%, sucrose 1%, pH 5.0.
[0034] The strains were inoculated in the α-L-rhamnosidase enzyme production medium with a conventional amount, and fermented for 120 hours at 45°C with a shaker speed of 100 rpm, and then all the fermented products were mixed at a speed of 8000 rpm Centrifuge for 10 minutes, collect the supernatant to obtain the crude enzyme solution of α-L-rhamnosidase;
[0035] (3) α-L-rhamnosidase catalyzes the directional biosynthesis of isoquercitrin by hydrolyzing rutin:
[0036] A. The free enzyme of ...
Embodiment 2
[0043] (1) Selection of strains: Select Penicillium CGMCC No. 2590 ( Penicillium notatum LJ-2) as a strain producing α-L-rhamnosidase;
[0044] (2) Preparation of crude enzyme solution of α-L-rhamnosidase:
[0045] Enzyme production medium formula (weight ratio): 1.5% potassium dihydrogen phosphate, 1.5% potassium dihydrogen phosphate, 1.5% ammonium sulfate, 0.5% sodium nitrate, 0.5% magnesium sulfate, 0.5% ferrous sulfate heptahydrate, zinc sulfate 0.5%, sucrose 5%, pH 8.0.
[0046] The strains were inoculated in the α-L-rhamnosidase enzyme production medium in a conventional amount, and fermented for 12 hours at 25°C with a shaker rotation speed of 250 rpm, and then all the fermented products were mixed at a rotation speed of 4000 rpm Centrifuge for 30 minutes, collect the supernatant to obtain the crude enzyme solution of α-L-rhamnosidase;
[0047] (3) α-L-rhamnosidase catalyzes the directional biosynthesis of isoquercitrin by hydrolyzing rutin:
[0048] A. The free e...
Embodiment 3
[0055] (1) Selection of strains: Select Aspergillus niger CGMCC No.2588 ( Aspergillus niger LJ-1) as a strain producing α-L-rhamnosidase;
[0056] (2) Preparation of crude enzyme solution of α-L-rhamnosidase:
[0057] Enzyme production medium formula is (weight ratio): dipotassium hydrogen phosphate 1.0%, potassium dihydrogen phosphate 0.5%, ammonium sulfate 0.5%, sodium nitrate 0.05%, magnesium sulfate 0.05%, ferrous sulfate 0.01%, zinc sulfate 0.01% , pH 6.0.
[0058] The strains were inoculated in the α-L-rhamnosidase enzyme production medium in a regular amount, and fermented for 72 hours at 35°C with a shaker rotation speed of 160 rpm, and then all the fermented products were mixed at a rotation speed of 5000 rpm Centrifuge for 20 minutes, collect the supernatant to obtain the crude enzyme solution of α-L-rhamnosidase;
[0059] (3) α-L-rhamnosidase catalyzes the directional biosynthesis of isoquercitrin by hydrolyzing rutin:
[0060] A. The free enzyme of α-L-rhamnos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com