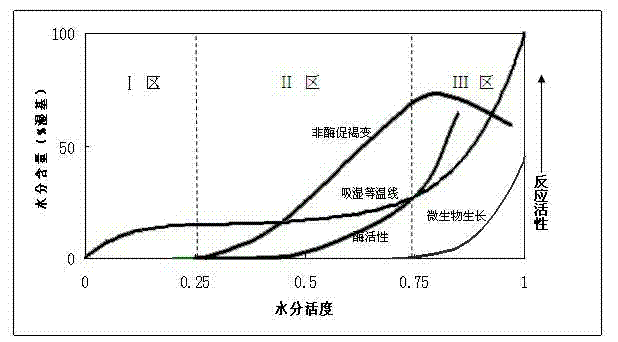
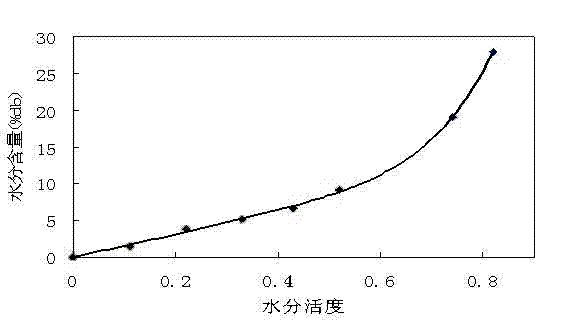
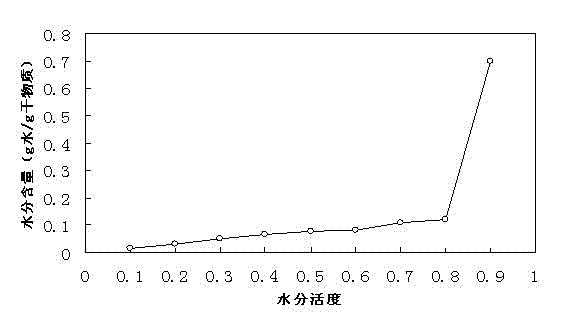
Effervescent medicinal preparation
A pharmaceutical preparation and preparation technology, which is applied in the field of solid instant pharmaceutical preparations, can solve problems such as poor effervescence effect and poor compressibility of glycine sodium carbonate, and achieve the effect of improving stability and reducing water activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-2
[0090] Ribavirin effervescent granules:
[0091] 1 2 Element Composition / unit, (mg) Composition / unit, (mg) Ribavirin 50 150 Glucose binders 300 290 Mannitol 600 600 Anhydrous citric acid 240 200 sodium bicarbonate 300 250 sodium saccharin 8 8 essence 2 2 Granule weight per bag 1500 1500
[0092] Note: Examples 1 and 2 are two different specifications of the same drug.
Embodiment 3-4
[0094] Ribavirin effervescent granules:
[0095] 3 4 Element Composition / unit, (mg) Composition / unit, (mg) Ribavirin 50 150 Povidone K30 80 80 Maltodextrin 100 100 Mannitol 1020 920 Anhydrous citric acid 340 340 sodium bicarbonate 400 400 stevia 8 8 essence 2 2 Granule weight per bag 2000 2000
[0096] Note: Examples 3 and 4 are two different specifications of the same drug.
Embodiment 5-6
[0098] Ribavirin effervescent granules:
[0099] 5 6 Element Composition / unit, (mg) Composition / unit, (mg) Ribavirin 50 150 Maltodextrin 800 700 sucrose 400 400 Anhydrous citric acid 340 340 sodium bicarbonate 400 400 sodium saccharin 8 8 essence 2 2 Granule weight per bag 2000 2000
[0100] Note: Examples 5 and 6 are two different specifications of the same medicine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com