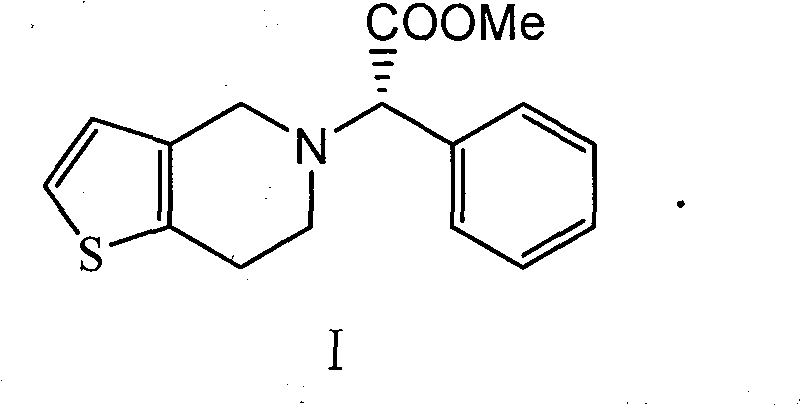
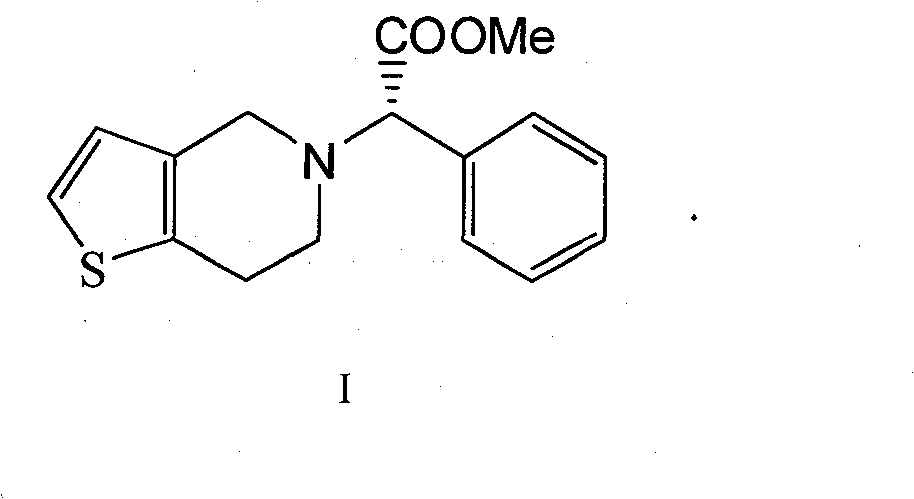
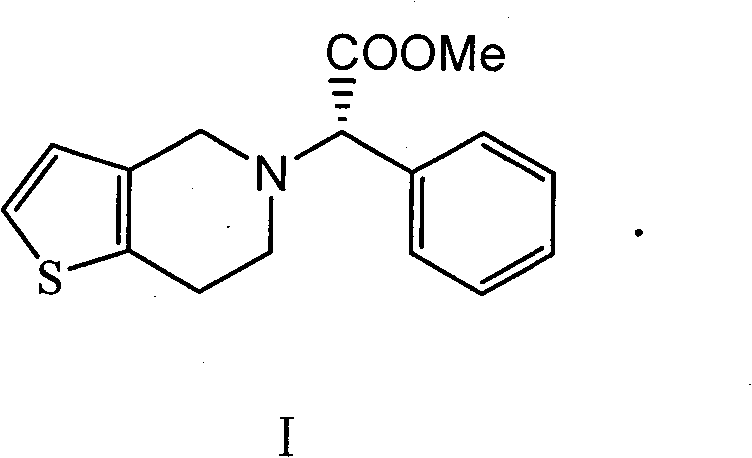
Preparation method of clopidogrel and salts thereof
A technology of clopidogrel and o-chloro, which is applied in the field of preparation of clopidogrel and its salts, and can solve the problems of complex reaction, low yield of clopidogrel and low optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] The synthesis of embodiment 1 (R)-o-chloromandelic acid methyl ester
[0014] Add 15 g of (R)-o-chloromandelic acid, 46.08 g of methanol, and 0.8 g of concentrated sulfuric acid into a 250 ml three-necked flask, and react under reflux for 3 hours. After the completion of the reaction, it was naturally cooled to room temperature, and the reaction system was heated with 10% K 2 CO 3 The solution was neutralized to neutral, the solvent was evaporated to dryness under reduced pressure, 100ml of dichloromethane was added, and 100ml×3 saturated NaHCO 3 The solution was washed, the organic phases were combined, and the solvent was evaporated to dryness under reduced pressure to obtain 15.9 g of relatively pure (R)-methyl o-chloromandelate, with a yield of 99% and an optical purity of 99.8%.
Embodiment 2
[0015] The synthesis of embodiment 2α-chloro-(2-chlorophenyl) methyl acetate
[0016] Add 20 g of (R)-methyl-o-chloromandelate and 17.8 g of thionyl chloride into a 250 ml three-neck flask, stir at room temperature for 16 hours, then heat and reflux for 2 hours. After the reaction is complete, pour the reaction solution into 100ml of ice water, stir until no gas is produced, extract with 100ml×2 dichloromethane, combine the organic phases, and wash the organic phases with saturated NaHCO 3 The solution and water were washed once respectively, and the solvent was evaporated to dryness under reduced pressure to obtain 21.1 g of a light yellow oily liquid with a yield of 97% and an optical purity of 99.6%.
Embodiment 3
[0017] Example 3 Synthesis of Clopidogrel
[0018] Add 219.06 g of α-chloro-(2-chlorophenyl) methyl acetate, 210.8 g of 4,5,6,7-tetrahydrothieno[3,2c]hexahydropyridine hydrochloride, and K 2 CO 3 276.4g, DMF1.3L, heated to 90°C, and kept for 4 hours. After the reaction was completed, the solid was filtered off, 6L of dichloromethane was added to the filtrate, washed with 6L×3 water, and the organic phase was spin-dried to obtain 310 g of clopidogrel free base with a yield of 92.3% and an optical purity of 99.4% ee.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com