Detection method of Chinese patent medicines containing at least two kinds of Radix Paeoniae Alba, Ginseng, Danshen, Artemisia annua, Licorice, Chuanxiong and Angelica
A detection method and technology of Chinese patent medicine, applied in the field of detection of active ingredients of traditional Chinese medicine, can solve the problems of wide range of medicinal materials, internal quality of difficult Chinese medicine pills, complex chemical components, etc., and achieve objective and accurate identification results, accurate and stable results, The effect of eliminating noise peaks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
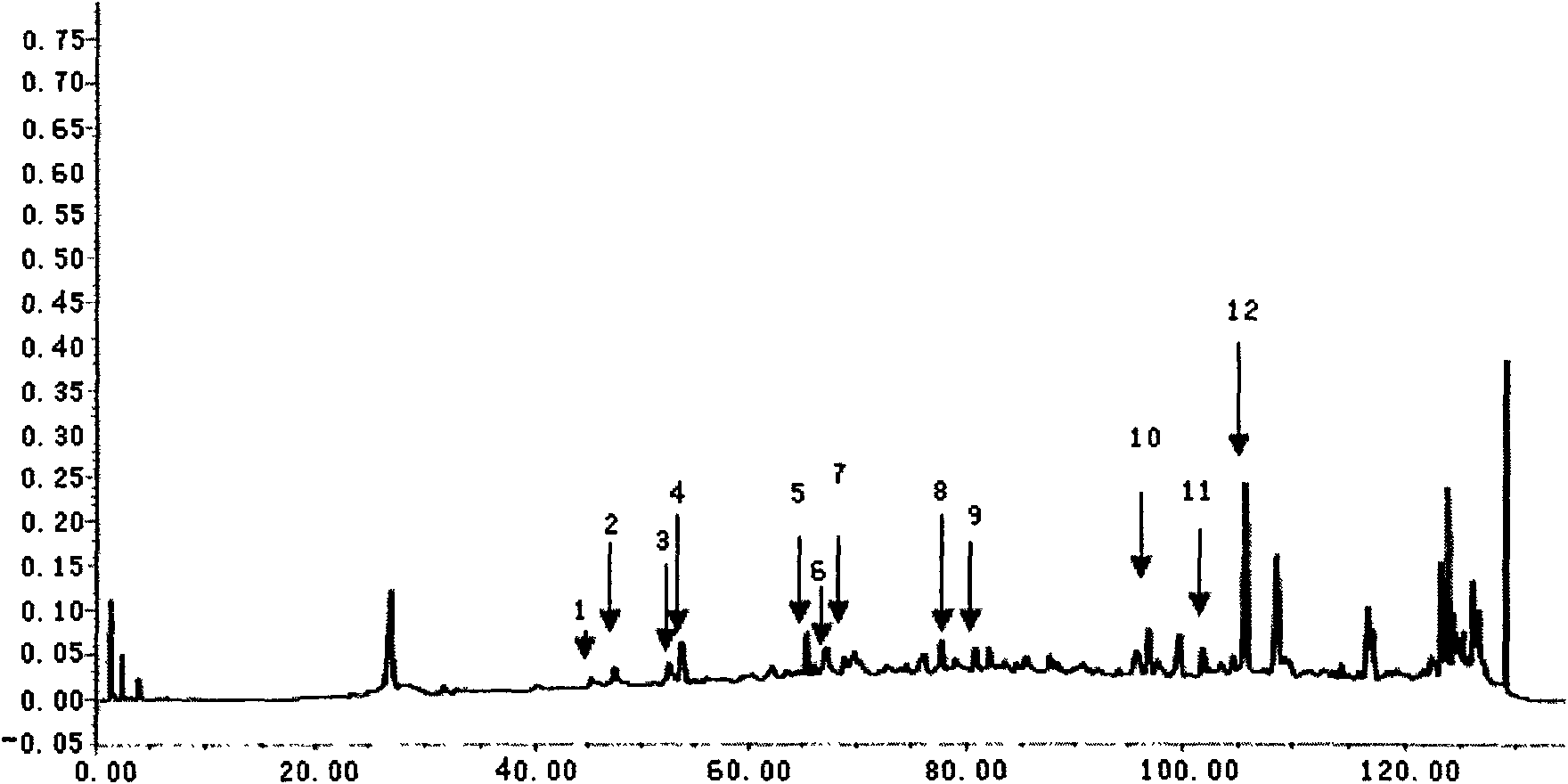
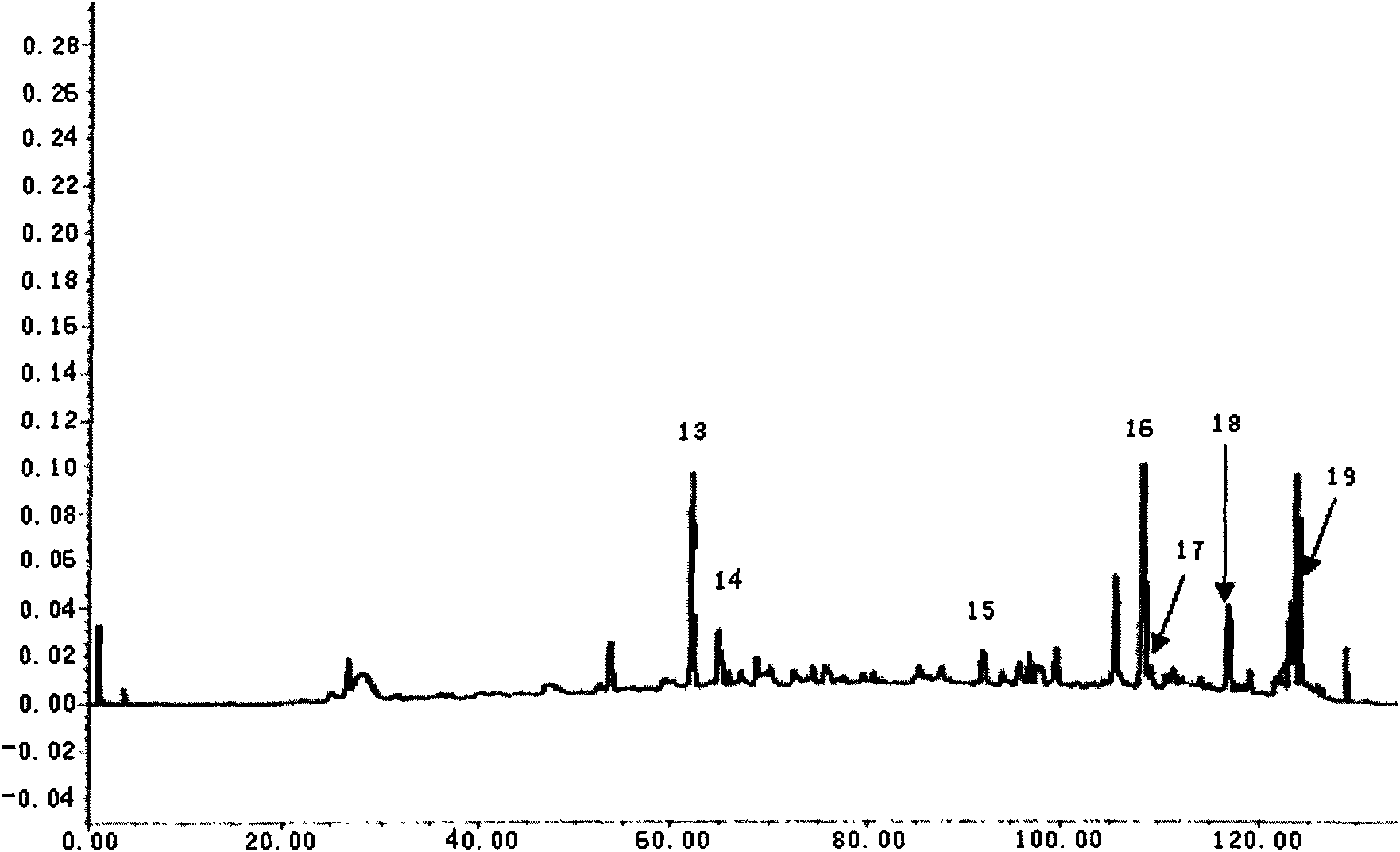
[0082] Example 1. The quality detection method of Tongren Wuji Baifeng Pills (Darmi Pills, Tongrentang Pharmaceutical)
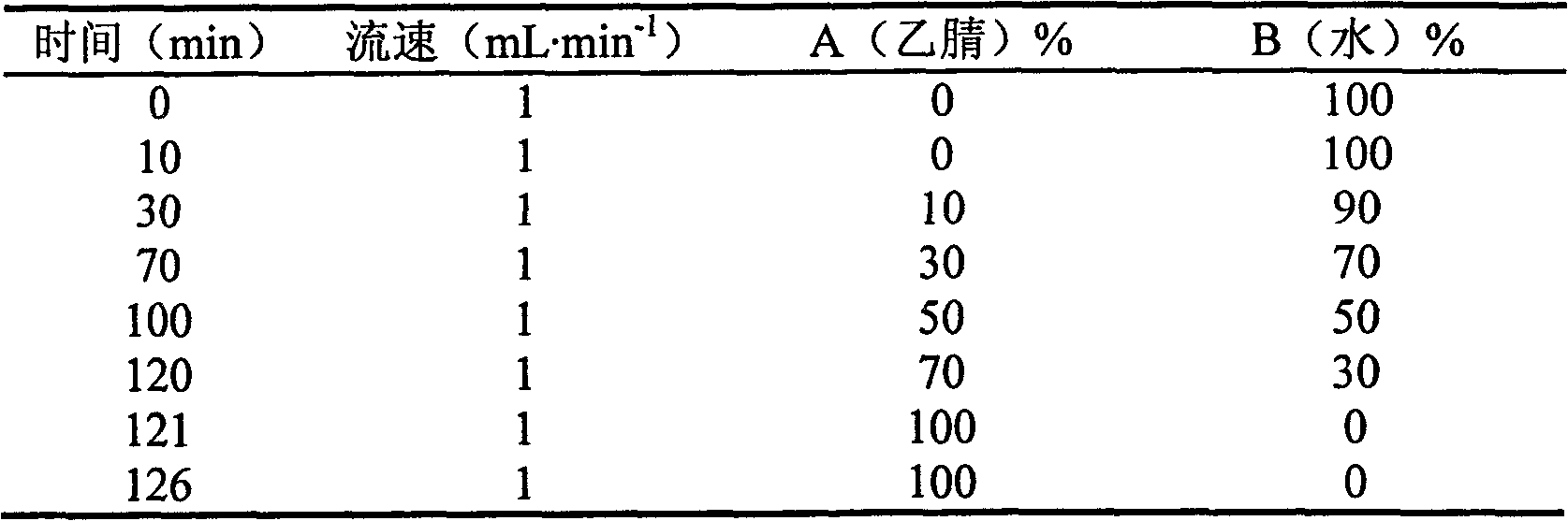
[0083] Chromatographic conditions and system suitability test: octadecylsilane bonded silica gel is used as filler; acetonitrile is used as mobile phase A, water is used as mobile phase B, and the gradient elution is carried out in the following table; the detection wavelength is 203nm (white peony , ginseng, licorice, Artemisia annua medicinal materials), 280nm (salvia miltiorrhiza, Chuanxiong, angelica medicinal materials).
[0084] Table HPLC mobile phase gradient
[0085]
[0086] Preparation of the test solution: Take an appropriate amount of this product, cut it into pieces, add an equal amount of diatomaceous earth and grind it evenly, accurately weigh 12g, add methanol 50mL, ultrasonically treat (power 300W, frequency 50kHz) for 30 minutes, let cool, and filter , the filtrate recovered the solvent and concentrated to dryness, the residue was diss...
Embodiment 2
[0092] Example 2. The detection method of active ingredients in Wuji Baifeng Pills (Shuiwan, Huiren Pharmaceutical)
[0093] 1. Preparation of the test solution: Accurately weigh Wuji Baifeng Pills (water pills 6g), add methanol equivalent to about 8 times the amount of the test sample, dissolve, preferably ultrasonically treat for 20-40 minutes, filter, and recover the solvent from the filtrate Concentrate to dryness, add about 50mL water to the residue to dissolve, pass through D101 macroporous resin adsorption column (the resin packed in the column is about 60g, inner diameter 2cm, length 12cm), elute with about 300mL water, discard the water, and use 25-35%wt of 50mL of ethanol for elution, discard the eluent, then use 75-85%wt (preferably 80%wt) of about 200mL of ethanol for elution, collect the eluent, evaporate to dryness, and wash the residue with methanol Dissolve and transfer to a measuring bottle (preferably a 5mL measuring bottle), add methanol to the mark, pass th...
Embodiment 3
[0100] Example 3. Detection method of active ingredients in other traditional Chinese medicine preparations containing two or more medicinal ingredients from Radix Paeoniae Alba, Ginseng, Salvia, Artemisia annua, Radix Glycyrrhizae, Rhizoma Chuanxiong, Angelica
[0101] 1. Preparation of the test solution: Accurately weigh the test product (should contain Radix Paeoniae Alba, Ginseng, Salvia miltiorrhiza, 6g), add methanol equivalent to about 8 times the amount of the test product, dissolve, filter , the filtrate is recovered and the solvent is concentrated to dryness, the residue is dissolved by adding about 50mL of water, passed through a D101 type macroporous resin adsorption column (about 60g of resin packed in the column), eluting with about 300mL of water, discarding the water, and then using 25- Elute with 50 mL of ethanol at 35% wt, discard the eluate, and then elute with about 200 mL of ethanol at 75% wt, collect the eluate, evaporate to dryness, dissolve the residue w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com