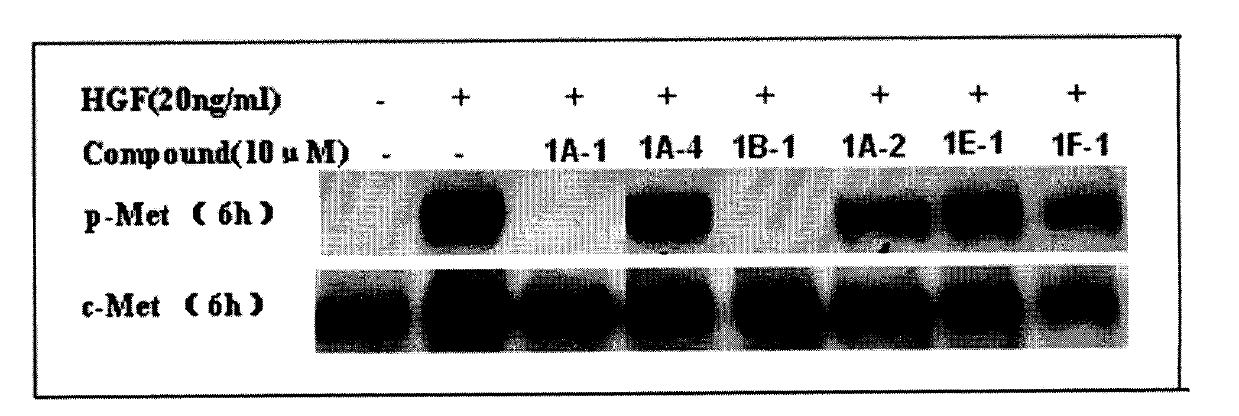
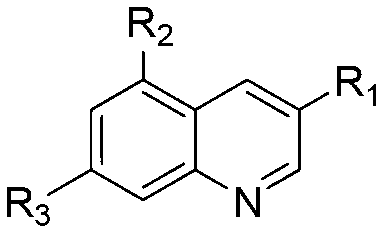
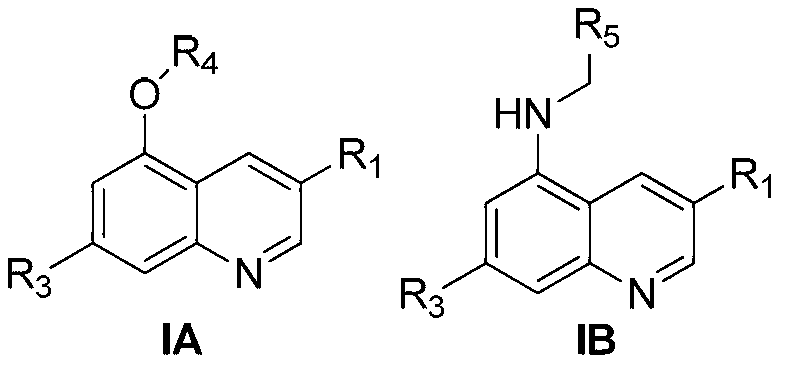
Quinoline compound and preparation method thereof, medicament combination containing compound and application of compound
A technology of compounds and quinolines, applied in the fields of quinoline compounds, their preparation, pharmaceutical compositions containing the compounds, and uses of the compounds, capable of solving problems such as limited structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0166] Preparation of Example 1 Intermediate [3-(4-methylpiperazin-1-yl)-7-trifluoromethyl-5-hydroxyquinoline]
[0167]
[0168] Preparation of 7-trifluoromethyl-5-nitroquinoline: 3-nitro 5-aminobenzotrifluoride (7.5g), glycerol (12.3g), and arsenic pentoxide (6.8g) were added to the reaction flask After stirring until the mixture is uniform, slowly add concentrated sulfuric acid (8g), first react at 130°C for one hour, then raise the temperature to 180°C for four hours. After the reaction is completed, the reaction solution is cooled to room temperature, and then adjusted to be alkaline with 4mol / L sodium hydroxide solution, the solid is filtered out, the solid is dissolved in ethanol, decolorized by activated carbon, the solvent is evaporated, and 7- Trifluoromethyl-5-nitroquinoline (1.7 g, 15%). 1 H-NMR (300MHz, CDCl 3 ): δ9.14(dd, 1H, J1=1.5Hz, J2=4.2Hz), 9.03(d, 1H, J=9.0Hz), 8.71(s, 1H), 8.54(d, 1H, J=1.5Hz ), 7.77 (dd, 1H, J1=9.0Hz, J2=4.2Hz).
[0169] The prepar...
preparation Embodiment 2
[0173] Preparation Example 2 Preparation of Compound IA-1:
[0174] The compound 3-(4-methylpiperazin-1-yl)-7-trifluoromethyl-5-hydroxyquinoline (16.0mg, 0.05mmol) was dissolved in THF (1ml), and Cs was added under ice-cooling 2 CO 3 (25.0mg, 0.078mmol), after stirring for 10min, m-nitrobenzyl bromide (11.6mg, 0.054mmol) was added to the reaction solution under nitrogen protection, the temperature was slowly raised to room temperature, stirred for two hours, concentrated and separated by column chromatography The target compound IA-1 (20.0 mg, 0.045 mmol) was purified with a yield of 90%.
[0175]
[0176] IA-1: 1 H-NMR (300MHz, CDCl 3 ): δ8.87(d, 1H, J=2.7Hz), 8.47(s, 1H), 8.25(d, 1H, J=9.3Hz), 7.93(s, 1H), 7.81(d, 1H, J= 7.5Hz), 7.73(d, 1H, J=2.4Hz), 7.63(t, 1H, J1=7.8Hz, J2=8.1Hz), 6.98(s, 1H), 5.37(s, 2H), 3.41(t , 4H, J1=4.8Hz, J2=5.1Hz), 2.64(t, 4H, J1=5.1Hz, J2=4.8Hz), 2.38(s, 3H).
preparation Embodiment 3
[0177] Preparation Example 3 Preparation of Compound IA-2:
[0178] Preparation of intermediate 3-(4-methylpiperazin-1-yl)-5-hydroxyquinoline:
[0179]
[0180] In addition to using 5-nitroquinoline instead of 7-trifluoromethyl-5-nitroquinoline, using the same method as in Preparation Example 1 to synthesize 3-(4-methylpiperazin-1-yl)-7- The same steps as trifluoromethyl-5-hydroxyquinoline synthesized 3-(4-methylpiperazin-1-yl)-5-hydroxyquinoline.
[0181] Preparation of compound IA-2:
[0182] The compound 3-(4-methylpiperazin-1-yl)-5-hydroxyquinoline (30.0mg, 0.123mmol) was dissolved in THF (1ml), and Cs was added under ice-cooling 2 CO 3(60.3mg, 0.185mmol), after stirring for 10min, m-fluorobenzyl bromide (25.5mg, 0.136mmol) was added to the reaction solution under nitrogen protection, the temperature was slowly raised to room temperature, stirred for two hours, concentrated and purified by column chromatography The target compound IA-2 (37.9 mg, 0.108 mmol) was obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com