Medicament formula and preparation thereof for preventing and curing altitude reaction and coronary heart disease
A technology for altitude sickness and coronary heart disease, applied in the direction of medical formula, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of less coronary heart disease and angina pectoris, and achieve the effect of high-quality selection, rapid altitude sickness, and alleviation of altitude sickness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0006] Example 1 - Preparation of Dropping Pills
[0007] (1) Extraction and purification process and conditions of salidroside
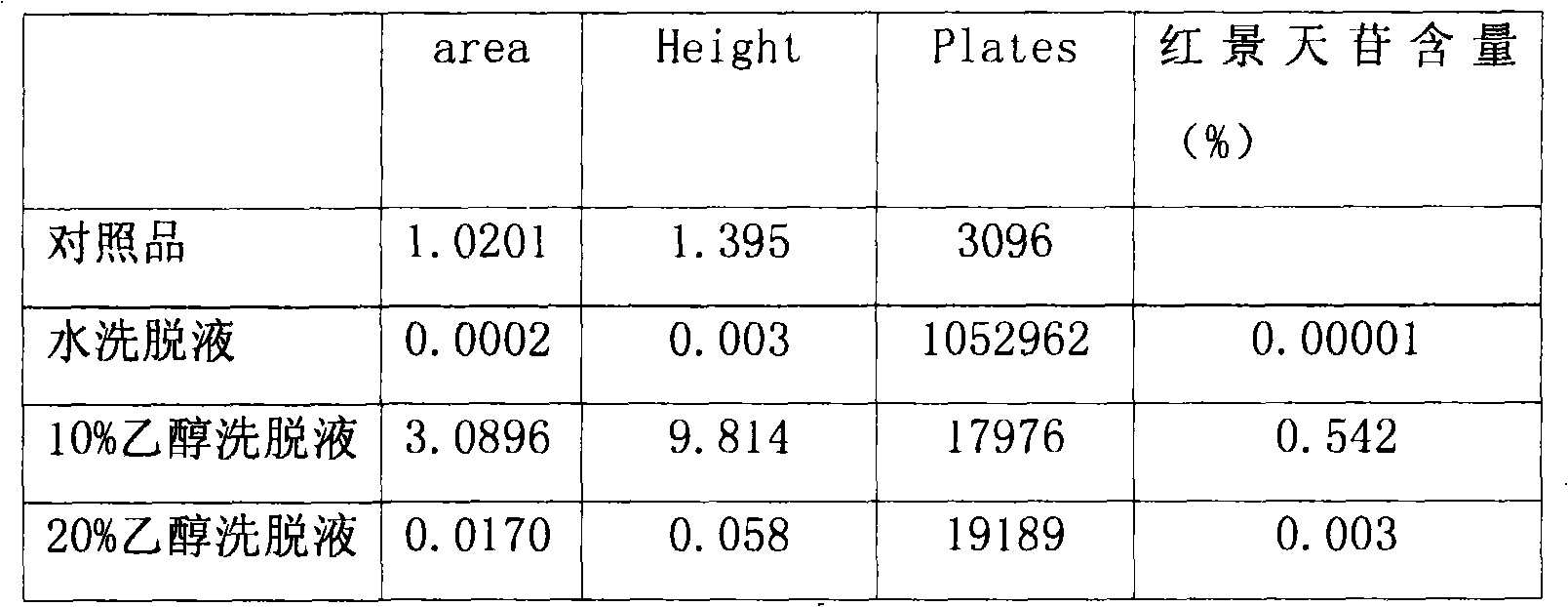
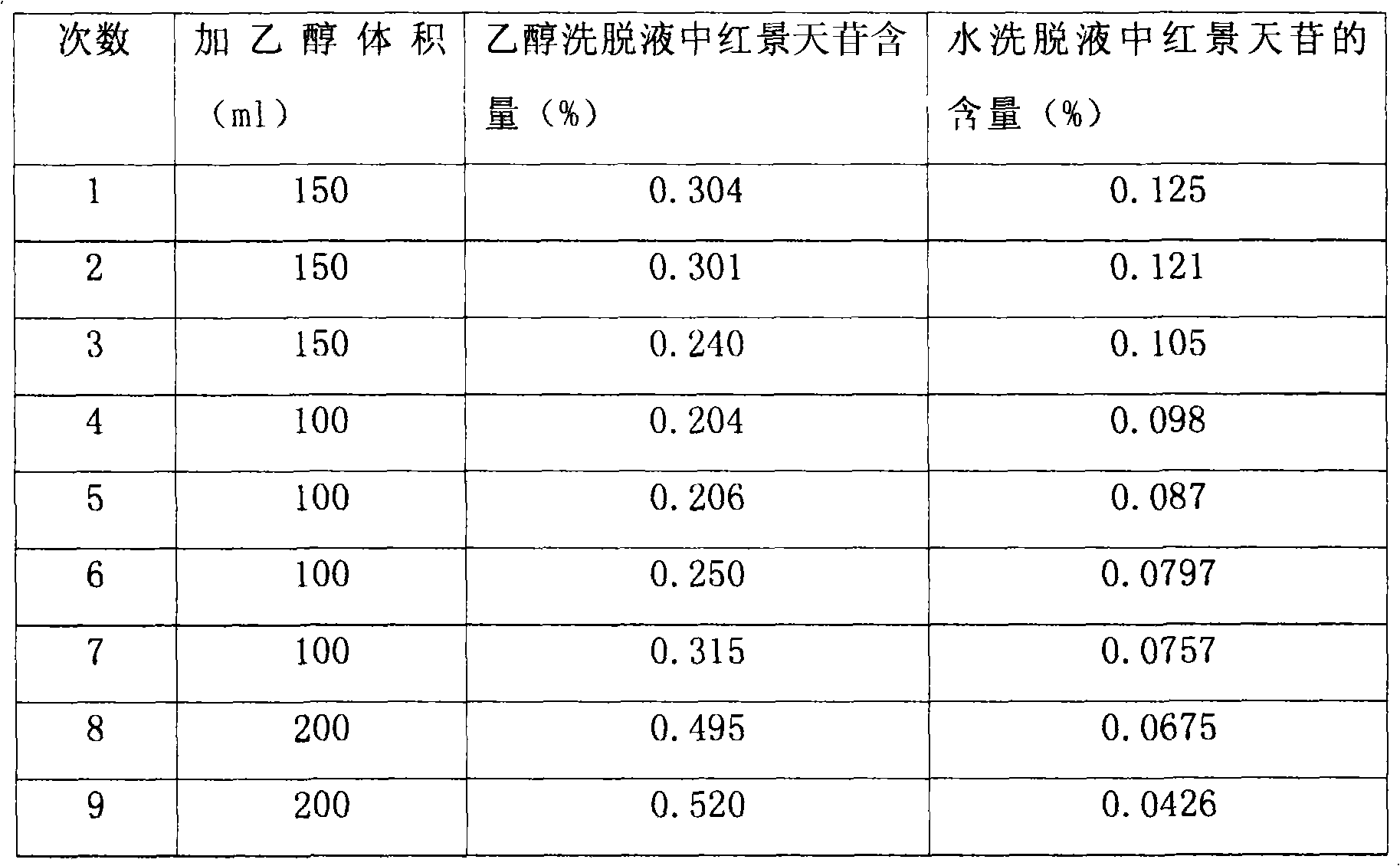
[0008] Take the prescribed amount of Rhodiola rosea, crush the Rhodiola rosea medicinal material, and pass through a No. 1 sieve. Add 70% ethanol according to the ratio of solid to liquid 1:8, heat the electric heating mantle to reflux and extract 3 times, each time for 1 hour, filter with a 200-mesh filter cloth, combine the filtrates, and recover the ethanol until it has no alcohol smell. Centrifuge the extract (centrifugal force: 3200, 10 min), take an appropriate amount of the supernatant and load the sample, first elute with 1 times the column volume of water, then elute with 50ml / 1g of 10% ethanol of medicinal materials, collect the 10% ethanol eluate, Concentrate to dryness. Purified salidolin was obtained.
[0009] research content:
[0010] Weigh 20g of Rhodiola rosea coarse powder, add 70% ethanol, according to the material-to-liquid r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com