Method for synthesizing high-purity alpha-calcium glycerophosphate
A technology of calcium glycerophosphate and synthesis method, applied in the direction of phosphorus organic compounds and the like, can solve the problems of high cost, complex synthesis process, difficult separation and the like, and achieve the effects of less impurity content, high purity and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
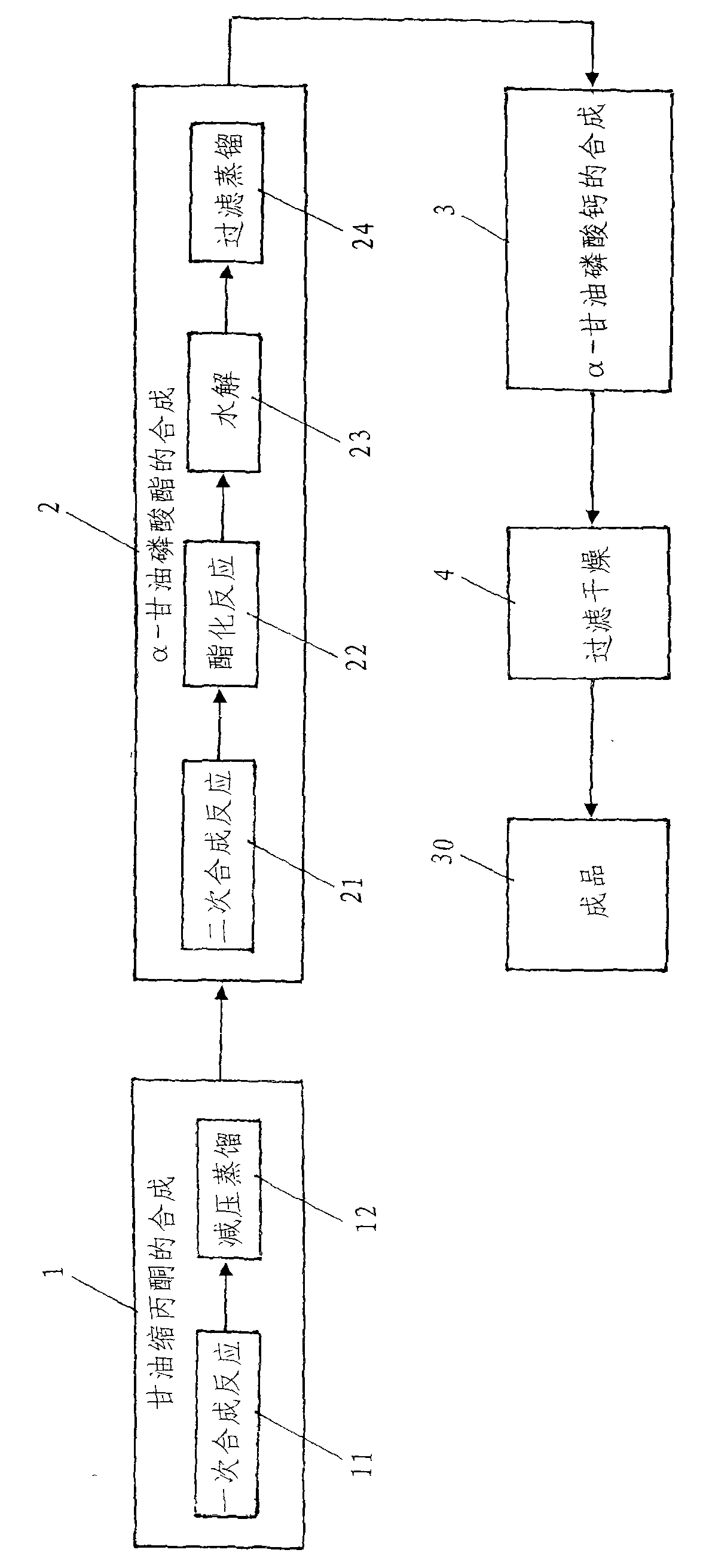
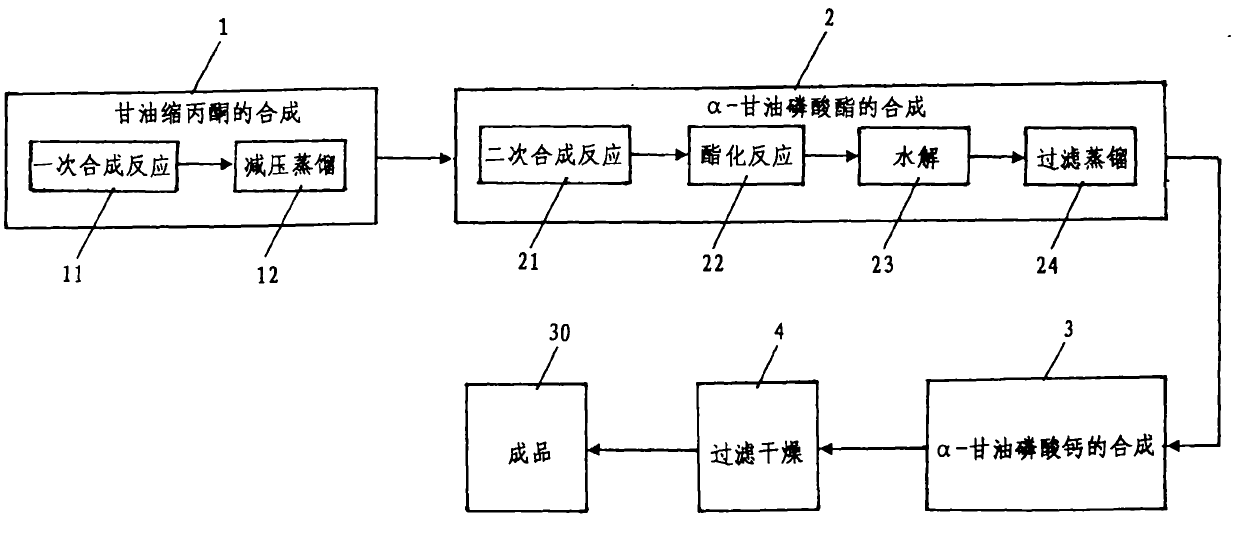
[0035] Such as figure 1 Shown, a kind of synthetic method of high-purity α-calcium glycerophosphate comprises the following steps:
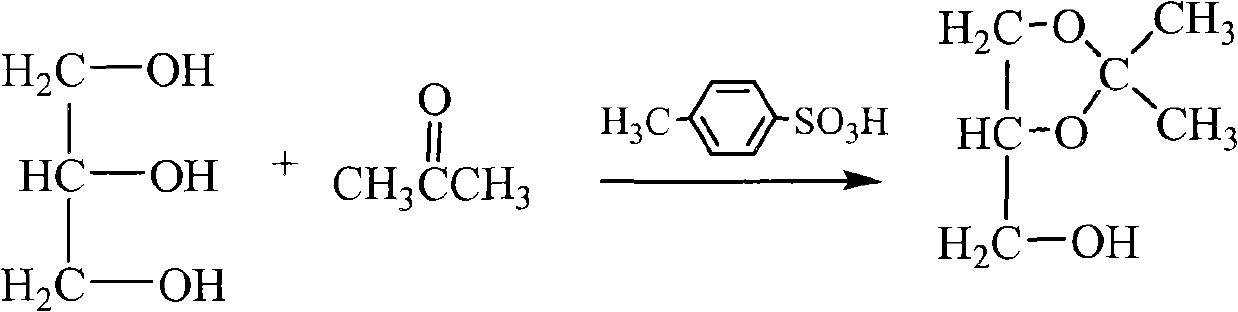
[0036] One, the synthesis of glycerol acetone
[0037] The synthetic 1 of glycerol acetone comprises a synthetic reaction 11 and underpressure distillation 12, and concrete steps are:
[0038] (i) One synthesis reaction
[0039] The raw materials are medical grade glycerin, reagent grade acetone and water-carrying agent, and the molar ratio is glycerol: acetone = 1: 2-4; the volume is 15-20 times of the theoretical water output, petroleum ether and 8%-10% of glycerin quality The catalyst is added to a three-port reaction kettle with a thermometer, a water separator and a reflux condenser, the magnetic stirring is turned on, the temperature is raised to reflux, and the water-carrying agent takes out the water generated by the reaction from the reaction system. When the amount of water in the water separator reaches the theoretical water output,...
Embodiment 1
[0061] Add 23.6g of glycerin, 38ml of acetone, 67.5ml of petroleum ether and 1.9g of p-toluenesulfonic acid into a three-port reaction kettle equipped with a thermometer, a water separator and a reflux condenser, turn on magnetic stirring, heat up to reflux, and Aqueous petroleum ether takes out the water generated by the reaction from the reaction system. When the amount of water in the water trap reached 4.5ml, the reaction was stopped. The reaction solution was distilled under reduced pressure, and fractions at 80° C. and 1 kPa were collected.
[0062] Add the glycerin acetal collected in the above steps into a three-port reaction kettle, and then add phosphorus pentoxide whose molar number is 3 / 4 of the glycerin acetal in batches under strong stirring, and ultrasonically and vigorously stir for 1 hour under the condition of an ice-water bath . The ultrasound was stopped, and the esterification reaction was carried out for 5 hours under the condition that the temperature ...
Embodiment 2
[0065] Add 23.6g of glycerin, 56ml of acetone, 81ml of petroleum ether and 2.1g of p-toluenesulfonic acid into a three-port reaction kettle equipped with a thermometer, water separator and reflux condenser, turn on magnetic stirring, heat up to reflux, and bring water The agent takes the water produced by the reaction out of the reaction system. When the amount of water in the water trap reached 4.5ml, the reaction was stopped. The reaction liquid was distilled under reduced pressure, and fractions of fractions at 81.5° C. and 1.33 kPa were collected.
[0066] Add the glycerin acetonide collected in the above steps into a three-hole reaction kettle, and then add phosphorus pentoxide whose molar number is 9 / 10 of the glycerol acetonide in batches under strong stirring, and ultrasonically and vigorously stir for 1.2 hours under the condition of an ice-water bath . The ultrasound was stopped, and the esterification reaction was carried out for 4 hours under the condition that the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com