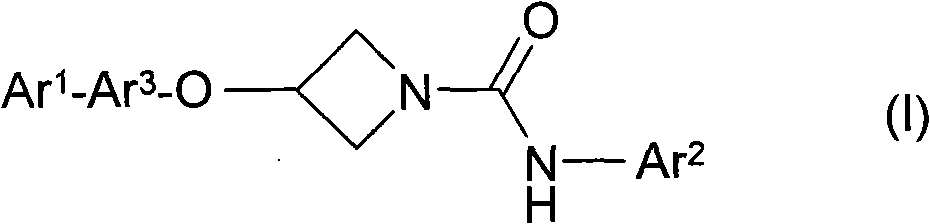
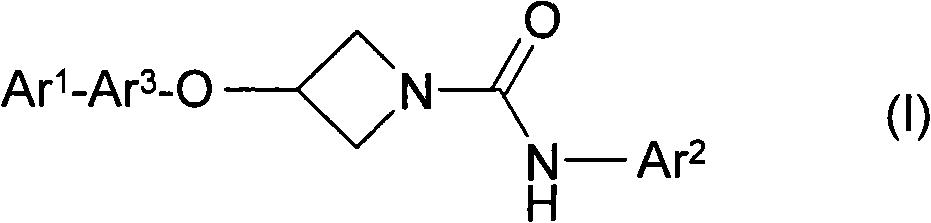
Azetidine derivatives
A single ring, compound technology, applied in the field of azetidine derivatives, can solve the problem of increasing the concentration of prostamide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1: 3-(Biphenyl-4-yloxy)-azetidine-1-carboxylic acid phenylamide
[0098]
[0099] step 1
[0100] 1-Benzhydryl-3-(biphenyl-4-yloxy)azetidine
[0101]
[0102] At room temperature, 4-phenylphenol (8.51 g, 50 mmol), 1-benzhydrylazetidin-3-ol (11.97 g, 50 mmol) and triphenylphosphine (13.11 g, 50 mmol) in acetonitrile (250 mL) was stirred for 20 minutes until all reactants were completely dissolved. Diisopropyl azodicarboxylate (9.84 mL, 10.11 g, 50 mmol) was added dropwise. A white precipitate formed and the initial reaction was mildly exothermic. The yellow color fades instantly. After 5 minutes, the reaction was heated to reflux temperature and stirred at this temperature for 3.25 hours when the precipitate dissolved. The mixture was cooled to room temperature and scraped with a spatula to induce crystallization. The mixture was cooled on ice and the solid was collected by filtration. The solid was further washed with cold acetonitrile and dried w...
Embodiment 2
[0109] Example 2: 3-(Biphenyl-4-yloxy)-azetidine-1-carboxylic acid (3-fluoro-phenyl)-amide
[0110]
[0111] The title compound was prepared as in Example 1, but using 3-fluorophenyl isocyanate instead of phenyl isocyanate. The obtained product is a white powder; LCMS retention time 2.61 minutes, m / z363.1[M+H] + ; 1 H nmr (400MHz; DMSO-d 6 )δ8.76 (s, 1H), 7.64-7.61 (m, 4H), 7.51-7.41 (m, 3H), 7.34-7.23 (m, 3H), 6.96 (d, 2H, J=8.4Hz), 6.77 -6.72 (m, 1H), 5.12-5.07 (m, 1H), 4.46 (dd, 2H, J = 9.2 and 6.4 Hz) and 3.94 (dd, 2H, J = 9.2 and 4.0 Hz).
Embodiment 3
[0112] Example 3: 3-(Biphenyl-4-yloxy)-azetidine-1-carboxylic acid (2-fluoro-phenyl)-amide
[0113]
[0114] The title compound was prepared following the procedure of Example 1, but using 2-fluorophenyl isocyanate instead of phenyl isocyanate. The product was purified by trituration with diethyl ether to afford the title compound as an off-white solid; LCMS retention time 2.60 min, m / z 363.1 [M+H] + ; 1 H nmr (400MHz; DMSO-d 6 )8.33(s, 1H), 7.64-7.58(m, 5H), 7.44(t, 2H, J=7.6Hz), 7.32(t, 1H, J=7.4Hz), 7.23-7.17(m, 1H), 7.14-7.08(m, 2H), 6.96(d, 2H, J=8.8Hz), 5.13-5.07(m, 1H), 4.45(dd, 2H, J=9.2 and 6.8Hz) and 3.94(dd, 2H, J = 9.2 and 3.6 Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com