Manufacturing method of drug vessel support with antibody immobilized on surface of support
A vascular stent and surface fixation technology, applied in the field of vascular stents, can solve the problems of promoting endothelial repair, no antibody binding active site, less antibody binding, etc., and achieve the effect of promoting endothelial cell repair and excellent treatment of restenosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
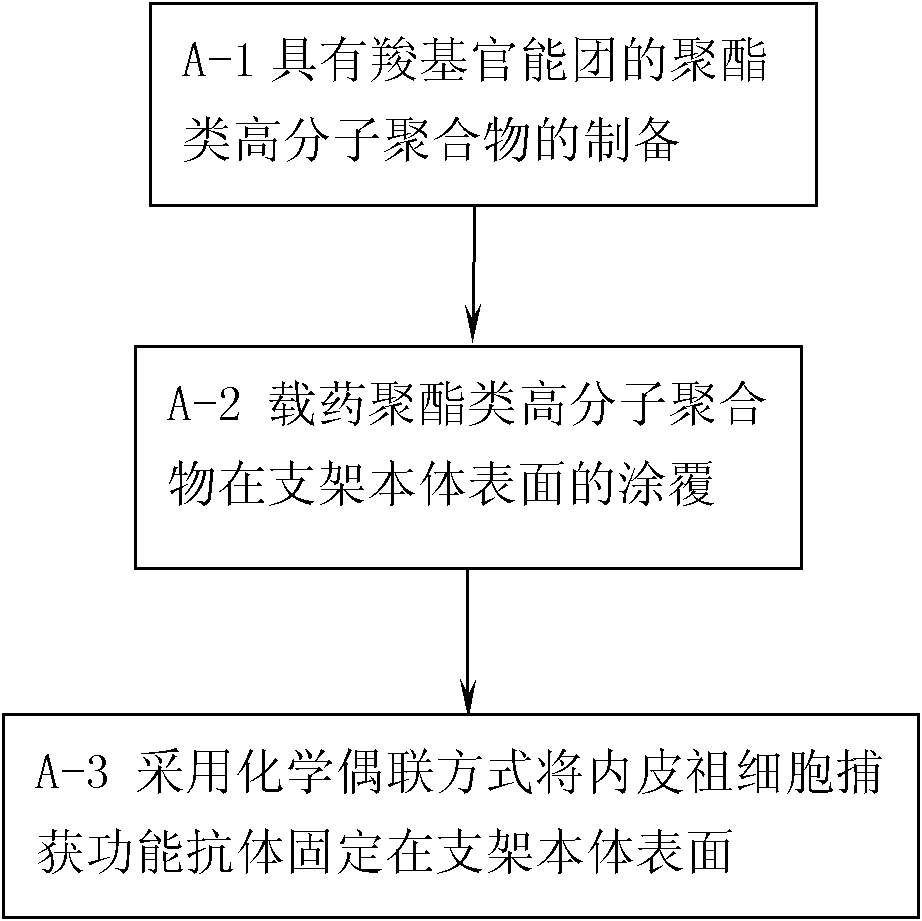
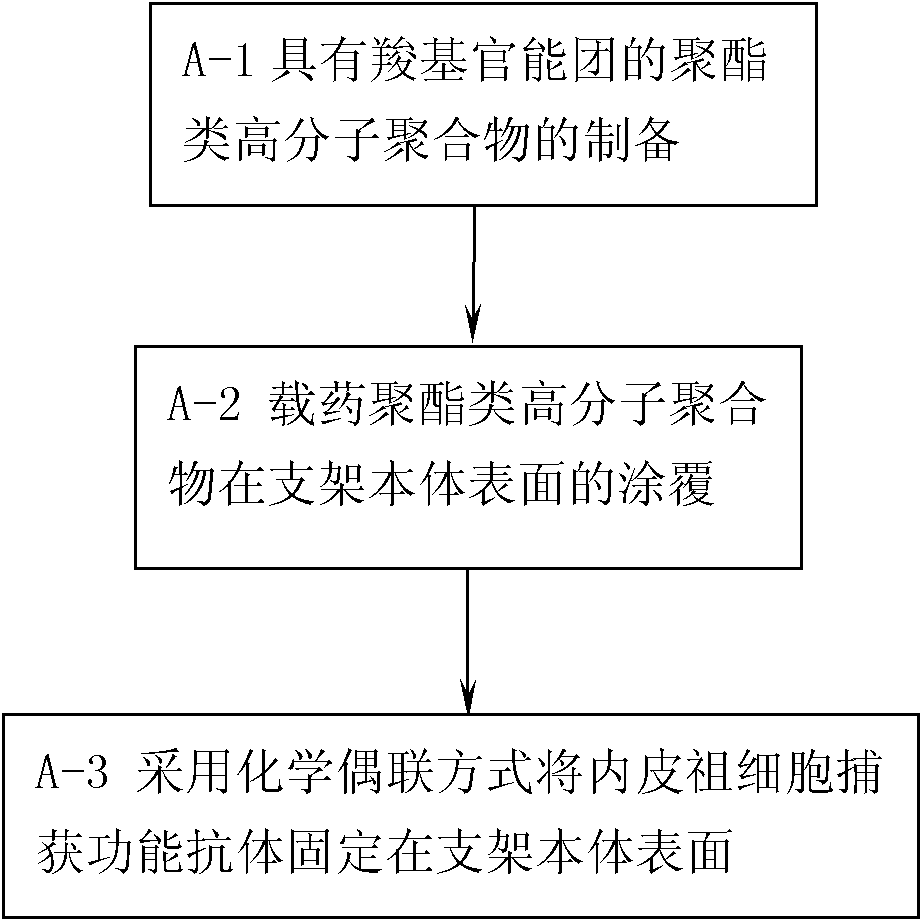
[0067] In Example 1, the material of the stent body is: stainless steel material; the selected polyester polymer is: polylactide glycolide, the selected anti-smooth muscle cell proliferation drug is paclitaxel, and the selected endothelial progenitor cells The capture antibody is CD133 antibody; the preparation process of a drug vascular stent with CD133 antibody and paclitaxel immobilized on the surface of the stent body is as follows:
[0068] Step A-1, preparation of polylactide glycolide with carboxyl functional groups:
[0069] According to the ratio of 1:15 in mass ratio, the polylactide glycolide is dissolved in the polymerization sealed tube equipped with dichloromethane, maleic anhydride and dibenzoyl peroxide are added to the above solution, and the The mass ratio of polylactide glycolide to maleic anhydride and dibenzoyl peroxide is 100:30:5, and the solvent is removed with a vacuum pump, and the vacuum is replaced with nitrogen for 3 times, and the tube is sealed a...
Embodiment 2
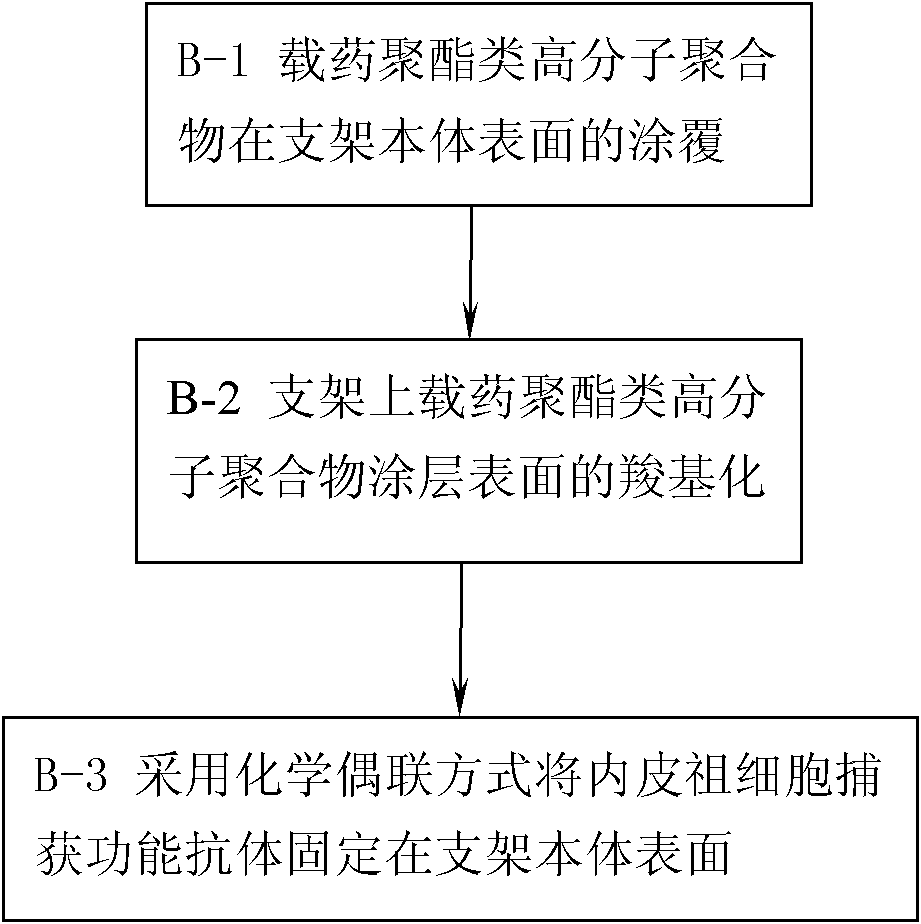
[0079] In Example 2, the material of the stent body is: stainless steel material; the selected polyester polymer is: polylactide, the selected anti-smooth muscle cell proliferation drug is rapamycin, and the selected endothelial progenitor cells The capture antibody is CD34 antibody; the preparation process of a drug vascular stent with CD34 antibody and rapamycin immobilized on the surface of the stent body is as follows:
[0080] Step A-1, preparation of polylactide with carboxyl functional groups:
[0081] According to the ratio of 1:40 in mass ratio, the polylactide is dissolved in the polymerization sealed tube containing dichloromethane, maleic anhydride and dibenzoyl peroxide are added to the above solution, and the polylactide The mass ratio with maleic anhydride and dibenzoyl peroxide is 100:40:8, remove the solvent with a vacuum pump, and replace it with nitrogen for 4 times, seal the tube at a vacuum of 80 Pa, and react for 48 hours at 100°C. Cool to room temperatu...
Embodiment 3
[0091] In Example 3, the material of the stent body is: stainless steel; the selected polyester polymer is: polyethylene glycol polylactide block polymer, and the selected anti-smooth muscle cell proliferation drug is dexamethasone Methasone, the selected endothelial progenitor cell capture antibody is CD31 antibody; the preparation process of a drug vascular stent with CD31 antibody and dexamethasone fixed on the surface of the stent body is as follows:
[0092] Step A-1, preparation of polyethylene glycol-polylactide block polymers with carboxyl functional groups:
[0093] According to the mass ratio of 1:5-40, the polyethylene glycol polylactide block polymer is dissolved in the polymerization sealed tube containing dichloromethane, and maleic anhydride and peroxide are added to the above solution. Dibenzoyl, the mass ratio of the polyethylene glycol polylactide block polymer to maleic anhydride and dibenzoyl peroxide is 100:5:1, and the solvent is removed with a vacuum pum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com