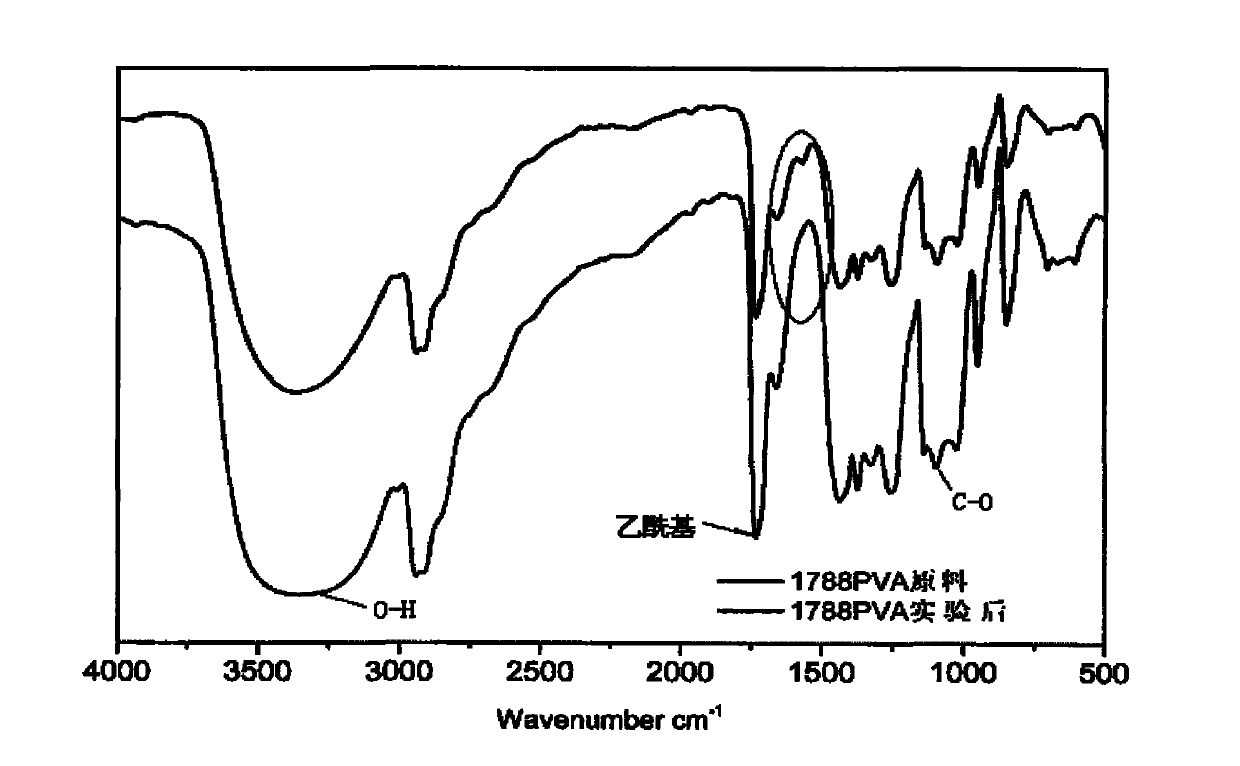
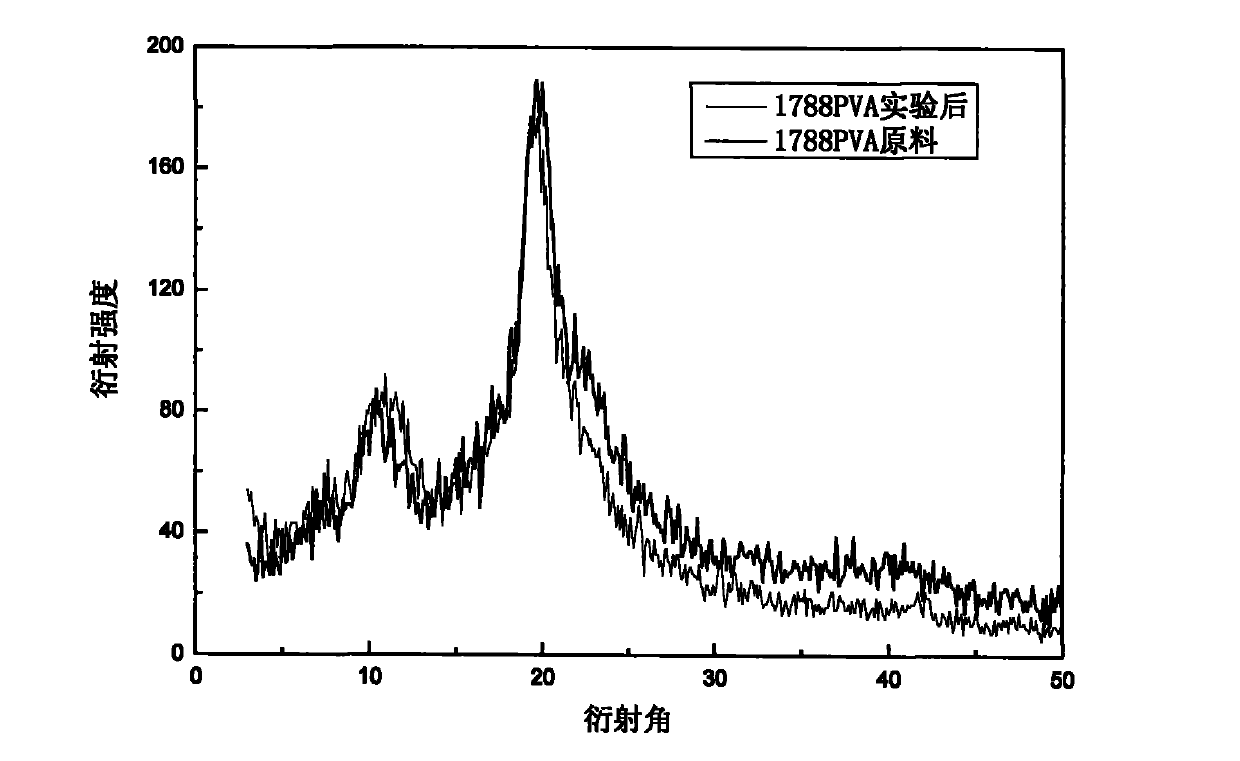
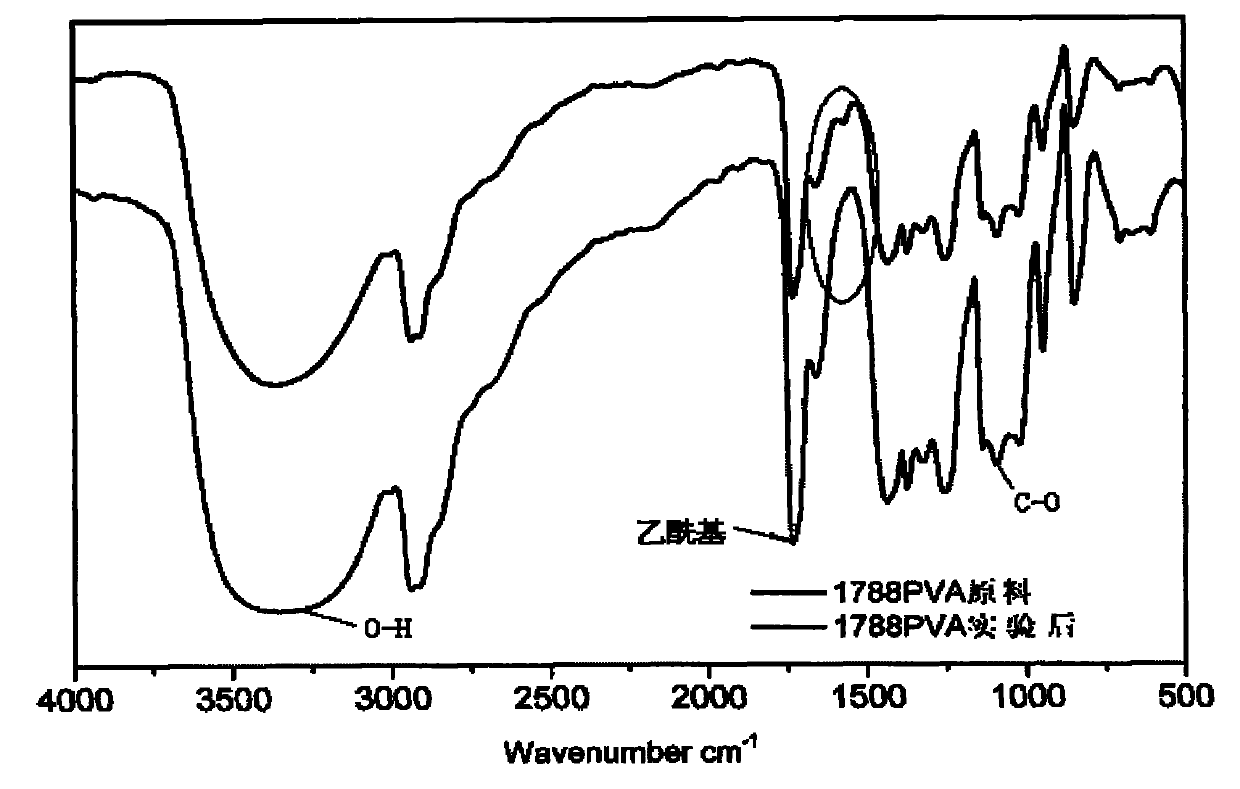
Purification method of polyvinyl alcohol
A technology of polyvinyl alcohol and purification method, applied in the field of purification of polyvinyl alcohol, can solve the problems of complex process, high energy consumption, increased cost and the like, and achieve the effect of reducing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Disperse 25 parts of polyvinyl alcohol particles (the degree of polymerization is 2400, and the degree of alcoholysis is 99%) in 75 parts of methanol, stir at 50°C for 20min, then filter and separate the polyvinyl alcohol, at 60°C Dry to obtain high-purity polyvinyl alcohol. According to the national standard (GB 12010.7-89), the ash content of polyvinyl alcohol before and after treatment was measured, and the results showed that the ash content of polyvinyl alcohol (polymerization degree: 2400, alcoholysis degree: 99%) dropped from 0.27% before treatment to 0.064%.
Embodiment 2
[0027] Example 2: disperse 30 parts of polyvinyl alcohol particles (the degree of polymerization is 2400, the degree of alcoholysis is 99%) into the mixed solution of 70 parts of methanol and water (methanol: water = 5: 1, by volume ratio) , stirred at 40°C for 30 minutes, then filtered to separate polyvinyl alcohol, and dried at 60°C to obtain high-purity polyvinyl alcohol. According to the national standard (GB 12010.7-89), the ash content of polyvinyl alcohol before and after treatment was measured, and the results showed that the ash content of polyvinyl alcohol (polymerization degree: 2400, alcoholysis degree: 99%) dropped from 0.27% before treatment to 0.043%.
Embodiment 3
[0028] Example 3: Disperse 30 parts of polyvinyl alcohol (the degree of polymerization is 1700, and the degree of alcoholysis is 88%) into 70 parts of ethyl acetate, stir at 60°C for 10min, then filter and separate the polyvinyl alcohol, 60°C Under drying, high-purity polyvinyl alcohol is obtained. According to the national standard (GB 12010.7-89), the ash content of polyvinyl alcohol before and after treatment was measured, and the results showed that the ash content of polyvinyl alcohol (polymerization degree: 1700, alcoholysis degree: 88%) dropped from 0.19% before treatment to 0.013%.
[0029] Certainly, in this example, water can also be used as a solvent to carry out purification treatment to polyvinyl alcohol.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ash content | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com