Serum maker of HBV (hepatitis B virus) infestor and application thereof
A serum marker, technology for infected people, used in biotechnology and medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
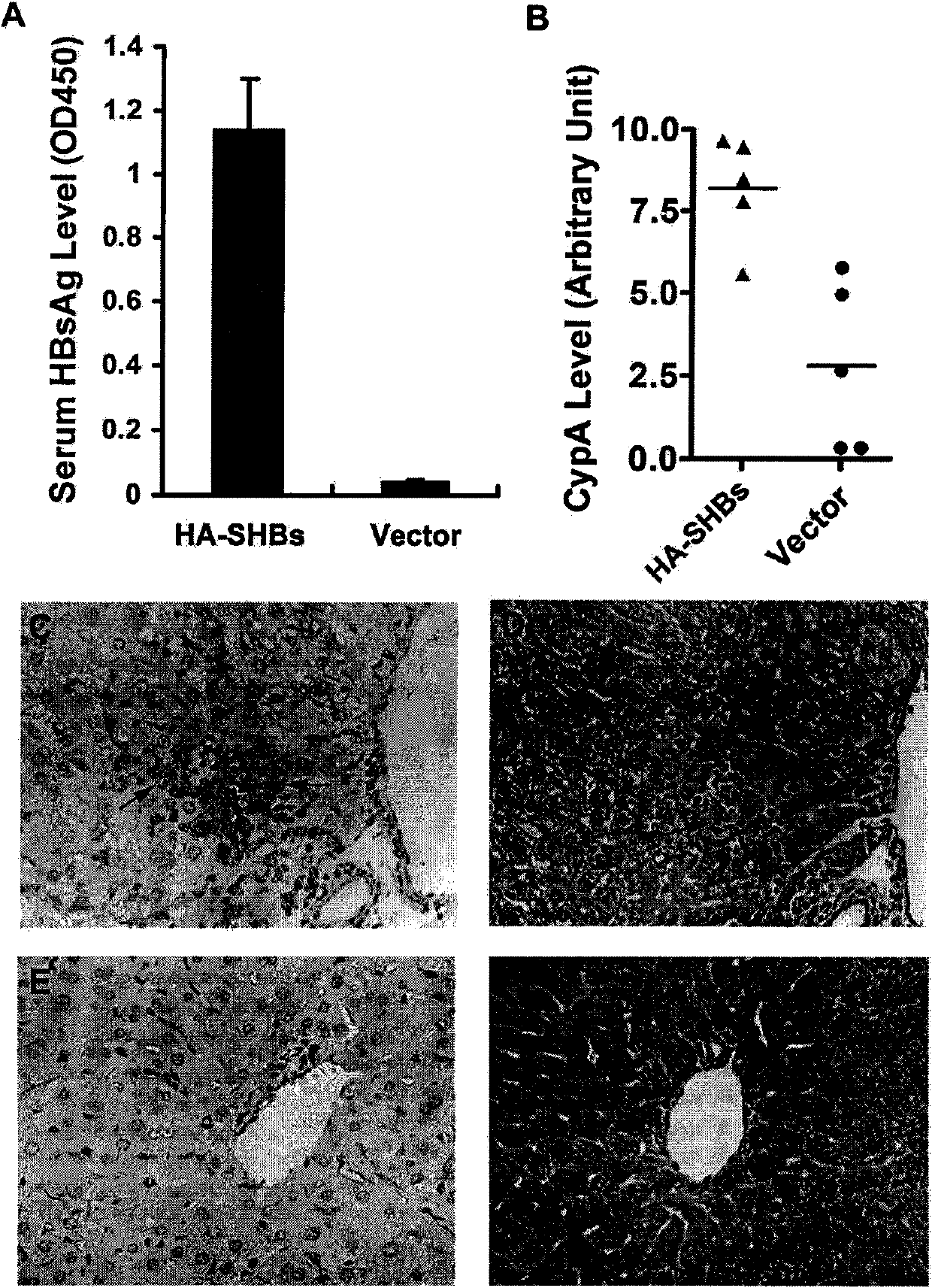
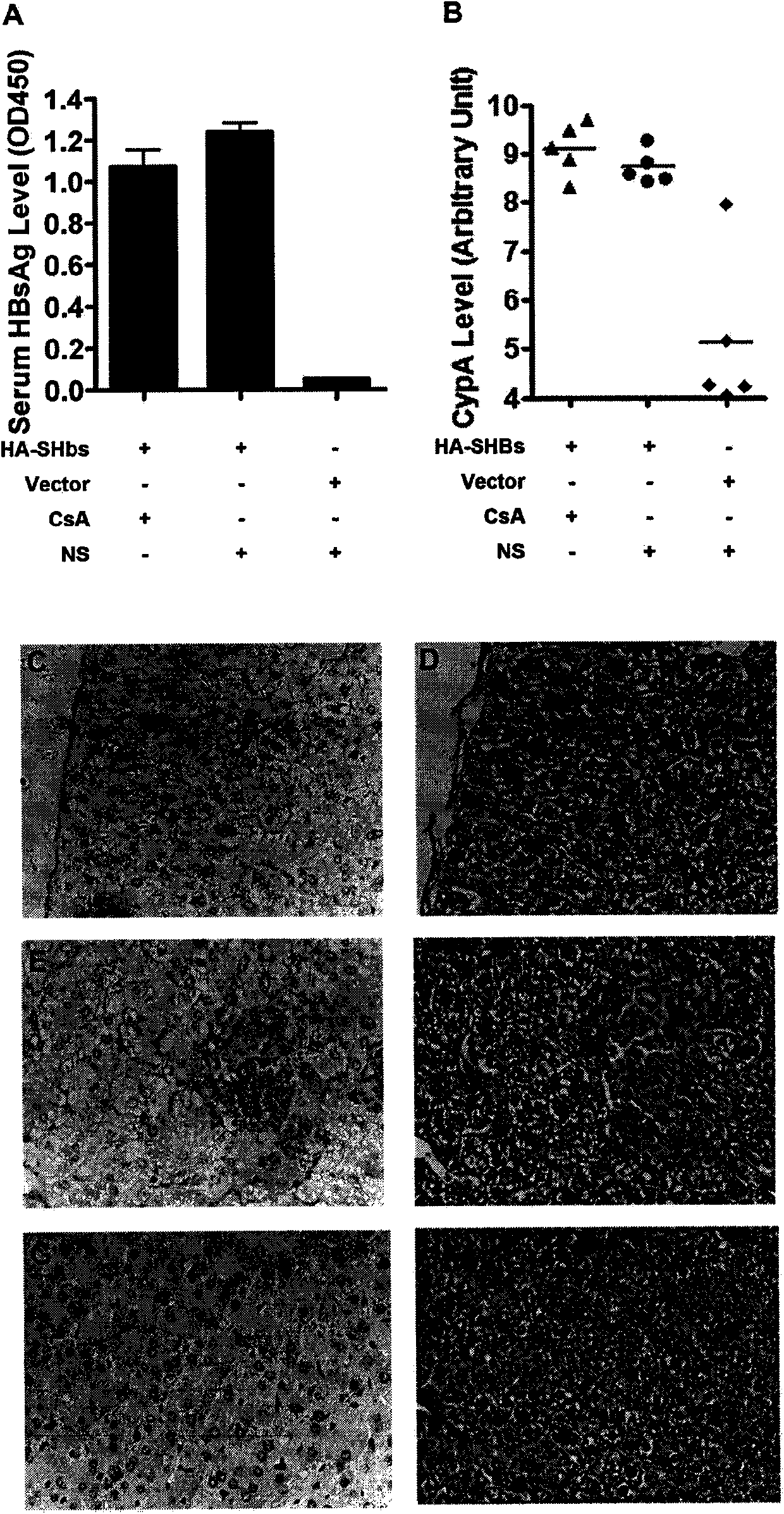
[0038] Embodiment 1 Animal Experimental Research
[0039] (1) Construction of HBsAg eukaryotic expression plasmid
[0040] According to the HBV whole genome sequence C8 (GenBank accession number AF461363) published in GenBank (http: / / www.ncbi.nlm.nih.gov / ) as a template, PCR primers were designed using known techniques (upstream primers were cta ggatcc atggagagcacaacatcag, downstream primer is gcc gaattc tcaaatgtatacccaaagac), using PCR technology to amplify the complete HBsAg coding sequence. Insert the amplified HBsAg gene fragment between the BamHI and EcoRI restriction sites in the multi-cloning site region of Invitrogen's pcDNA3.1 plasmid vector. The HBsAg gene expression is controlled by the CMV promoter and can be expressed in eukaryotic cells .
[0041] (2) Using known high-pressure tail vein injection technique to express HBsAg in mouse liver
[0042] Dilute 10 μg of HBsAg eukaryotic expression plasmid in Ringer’s isotonic buffer (154mM NaCl, 5.63mM KCl, 2.25mM ...
Embodiment 2
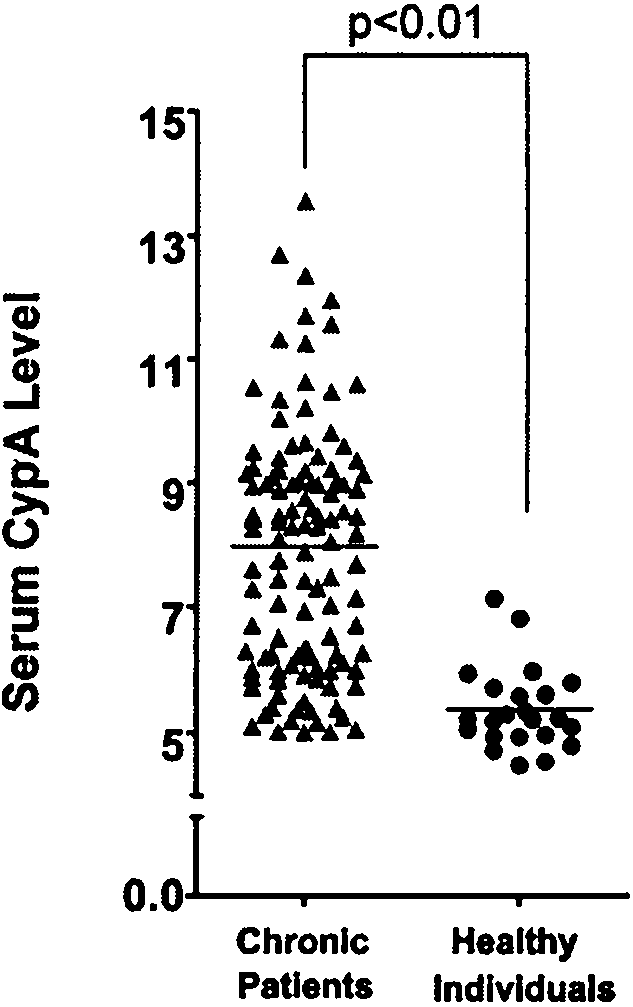
[0054] Example 2 Research on Serum CypA Content of Patients with Chronic Hepatitis B
[0055] 1. Collect the serum of chronic hepatitis B patients and healthy normal people
[0056] The criteria for the diagnosis of chronic hepatitis B are that the serum HBsAg has been positive for at least 6 months, the serum transaminase level has increased by at least 2 times, and it is accompanied by other corresponding symptoms. The corresponding control group is the general anti-group without hepatitis B virus infection and normal serum transaminase level. All samples were ruled out the possibility of hepatitis C (hepatitis Cvirus, HCV), hepatitis D virus (HDV), and human immunodeficiency virus (humanimmunodeficiency virus 1, HIV-1) co-infection. Take 1ul of serum and separate it by SDS-PAGE gel electrophoresis, then transfer the protein to the nitrocellulose membrane with 200mA constant flow for 1 hour, and then block with 5% skim milk powder solution at room temperature for 2 hours, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com