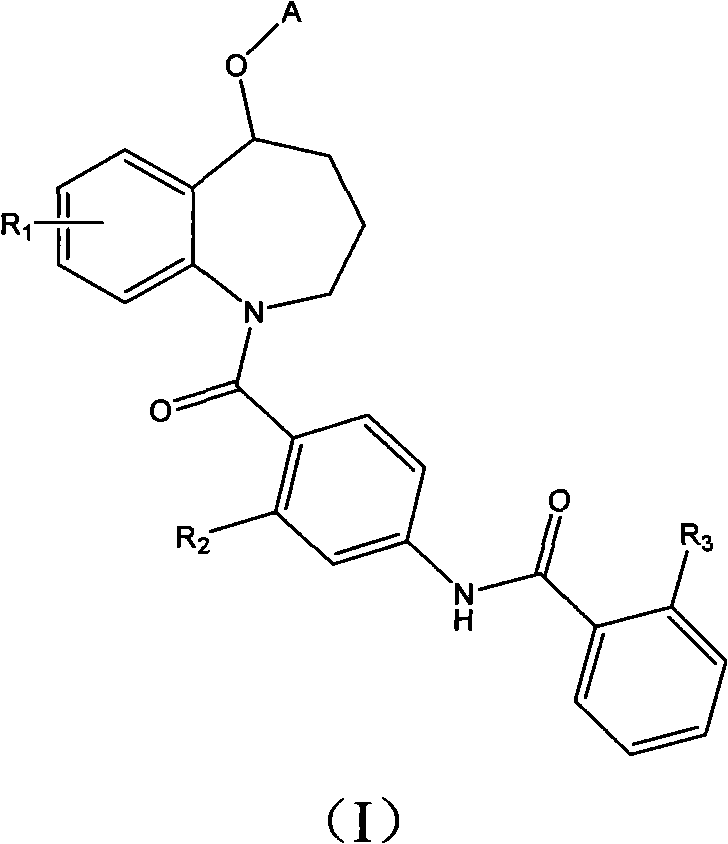
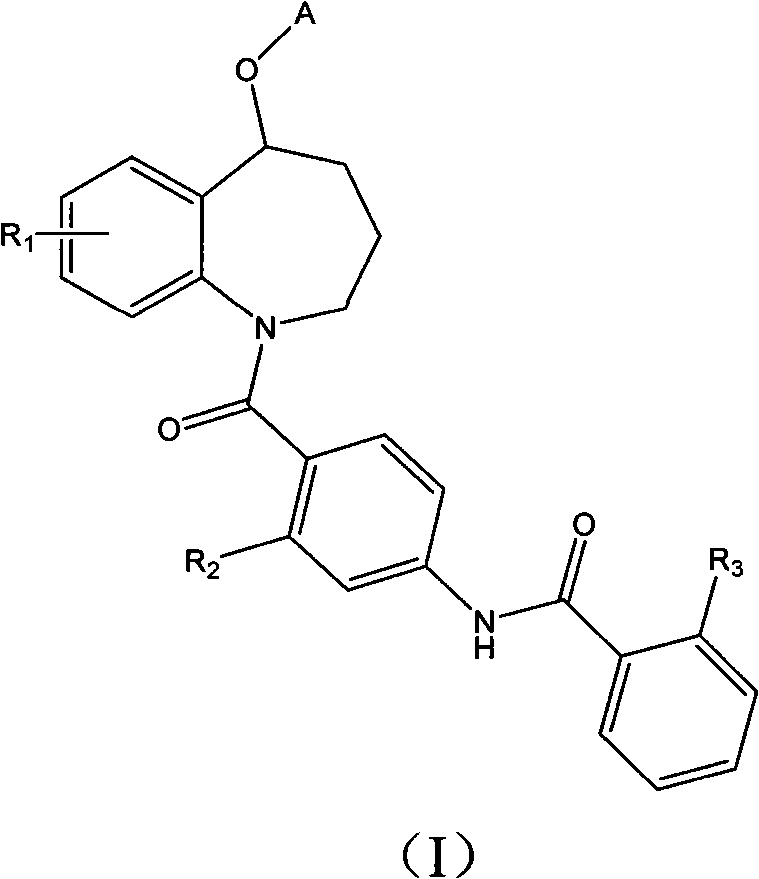
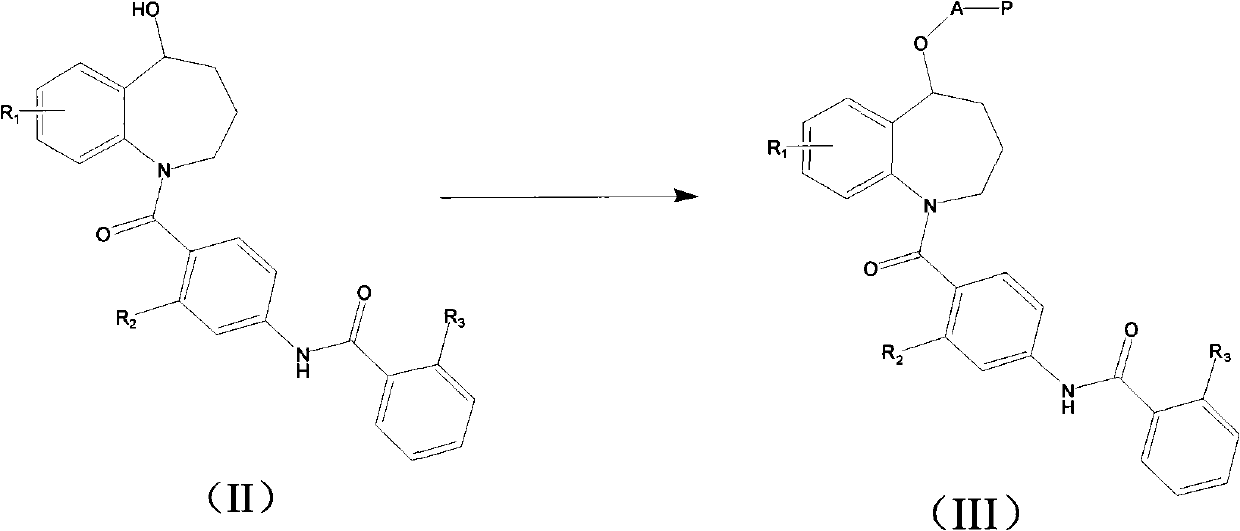
Benzazepines compounds serving as vasopressin receptor antagonism
A compound, benzoazepine technology, applied in the field of preparation of medicines for the treatment of vasopressin-related diseases, can solve the problems of difficulty in making injections, poor water solubility, etc., and achieve the effect of excellent water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] N-[4-[5-(2-aminoacetoxy)-2,3,4,5-tetrahydro-1-benzazepine-1-formyl]-benzene represented by formula (V) The preparation of base]-2-methylbenzamide hydrochloride
[0028]
[0029] Include the following steps:
[0030] 1) Preparation of N-[4-[5-hydroxyl-2,3,4,5-tetrahydro-1-benzazepine-1-formyl]-phenyl]-2-methylbenzamide
[0031] N-[4-[5-oxo-2,3,4,5-tetrahydro-1-benzazepine-1-formyl]-phenyl]-2-methylbenzamide 2 g In 10ml of methanol and 10ml of THF mixture, add 0.8g of sodium borohydride twice at room temperature, stir for 1 hour after the addition, concentrate to remove the solvent, add 30ml of water to the residue, stir for 1 hour, filter, and dry to obtain a light yellow powder, MS- ESI: 401.5 [M+H].
[0032] 2) Preparation of N-[4-[5-(2-(N-tert-butoxycarbonyl)aminoacetoxy)-2,3,4,5-tetrahydro-1-benzazepine-1-methyl Acyl]-phenyl]-2-methylbenzamide hydrochloride
[0033] Add 494 mg of the product in 1), 475 mg of BOC-glycine to 20 ml of dichloromethane, add 500 mg...
Embodiment 2
[0037] N-[4-[5-(2-amino-3-methylbutyryloxy)-2,3,4,5-tetrahydro-1-benzazepine-1 represented by formula (VI) Preparation of -formyl]-phenyl]-2-methylbenzamide hydrochloride
[0038]
[0039] Following the same method as in Example 1, except that glycine was replaced by valine, an off-white solid was obtained, melting point: 183-185° C., MS-ESI: 501.5 [M+H].
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com