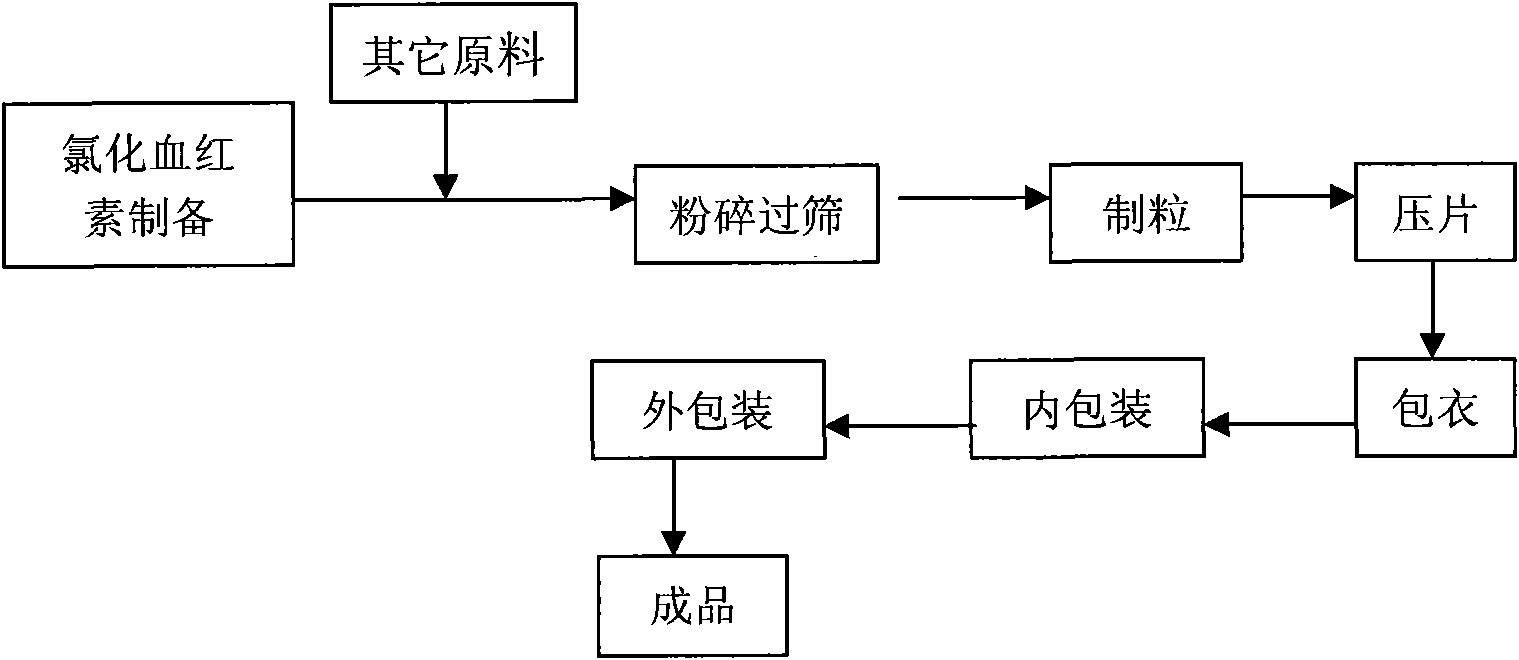
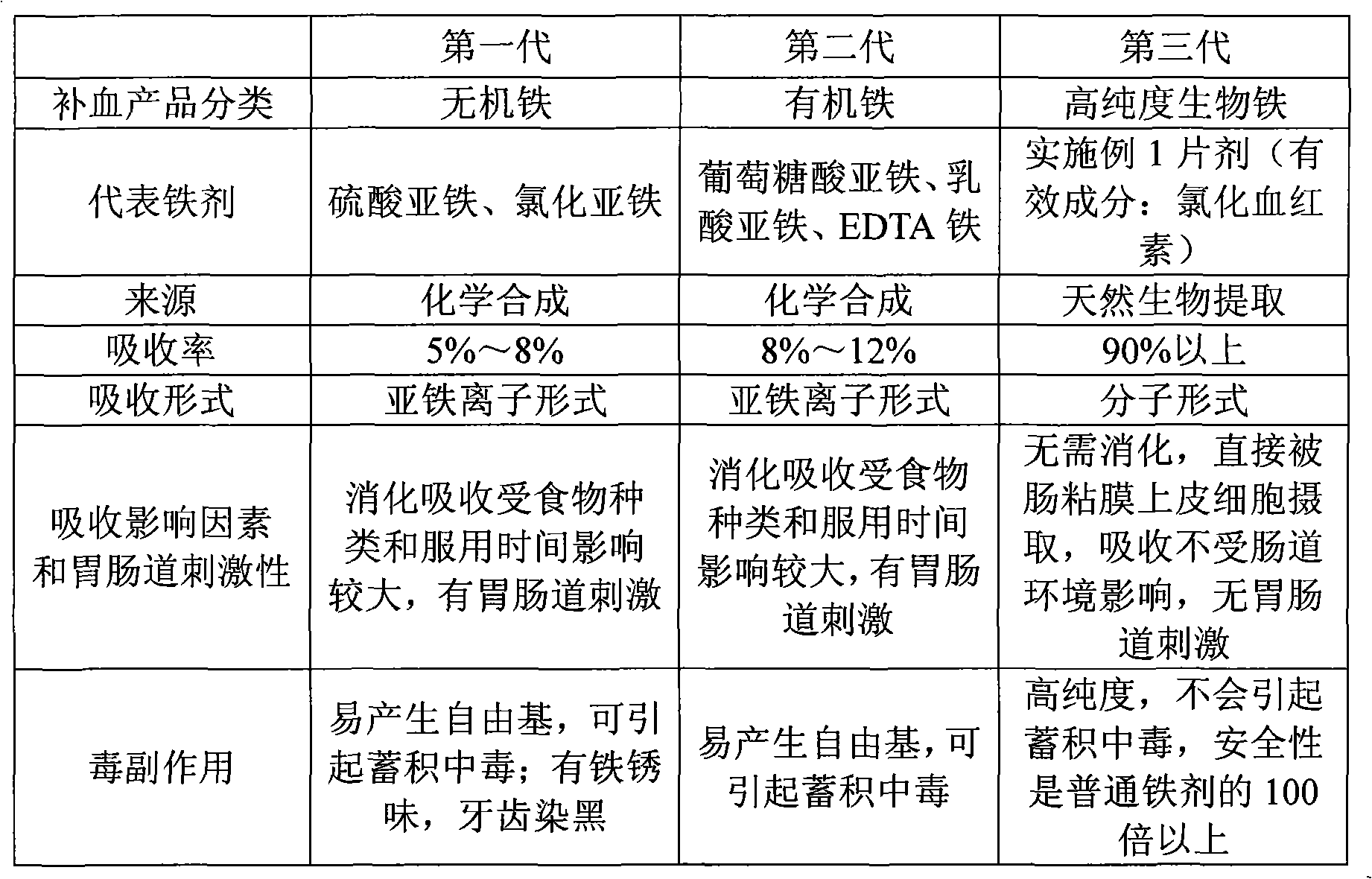
Chlorhematin and preparation method thereof as well as blood-enriching pharmaceutical composition
A technology of hemin and its composition, which is applied in the field of hemin and its preparation method and blood-enriching drug composition, and can solve the problems of toxic side effects and poor absorption of ionized iron, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 Preparation of hemin
[0025] 1. Digestion: Mix anticoagulated fresh pig blood and glacial acetic acid containing 1 (wt)% NaCl in a volume ratio of 1:5 in a reaction kettle, and digest at 115° C. for 4 hours.
[0026] 2. Enzymolysis: Put the fluid obtained after digestion and 20 (wt)% acetic acid solution into the reaction kettle at a volume ratio of 1:6, start stirring at a speed of 60 rpm, and after stirring for 30 minutes, adjust the pH value of the reaction solution to 3.5. Then 537 protease (purchased from Jiangmen Yadong Biochemical Co., Ltd.) was added at a ratio of 0.5 (wt)% (equivalent to 500 IU / ml reaction liquid), and stirred at 60° C. for 48 hours. Then discharge.
[0027] 3. Filtration and separation: The reaction solution after enzymolysis is first filtered with a filter press, and then separated with a separator, and the rotation speed is required to be 7600 rpm. Take the solid particles after separation.
[0028] 4. Drying: Bake the soli...
Embodiment 2
[0029] Embodiment 2 Preparation of hemin
[0030] 1. Digestion: Mix anticoagulated fresh pig blood with glacial acetic acid containing 0.5 (wt)% NaCl in a volume ratio of 1:7 in a reactor, and digest at 100° C. for 8 hours.
[0031] 2. Enzymolysis: Put the fluid obtained after digestion and 30 (wt)% acetic acid solution into the reaction kettle at a volume ratio of 1:8, start stirring, and the rotation speed is required to be 60 rpm. After stirring for 30 minutes, adjust the pH value of the reaction solution to 4.5. Add 537 protease at a ratio of 0.1 (wt) %, and stir at 50° C. for 72 hours. Then discharge.
[0032] 3. Filtration and separation: The reaction solution after enzymolysis is first filtered with a filter press, and then separated with a separator, and the rotation speed is required to be 7600 rpm. Take the solid particles after separation.
[0033] 4. Drying: Bake the solid particles at 121°C for more than 2 hours to constant weight to obtain hemin. The purity ...
Embodiment 3
[0034] Embodiment 3 Preparation of hemin
[0035] 1. Digestion: Mix anticoagulated fresh pig blood with glacial acetic acid containing 2 (wt)% NaCl in a volume ratio of 1:3 in a reaction kettle, and digest at 130° C. for 1 hour.
[0036] 2. Enzymolysis: Put the fluid obtained after digestion and 10 (wt)% acetic acid solution into the reaction kettle at a volume ratio of 1:4, start stirring at a speed of 60 rpm, and after stirring for 30 minutes, adjust the pH value of the reaction solution to 4.0. Add 537 protease at a ratio of 1 (wt)% and stir at 70° C. for 36 hours. Then discharge.
[0037] 3. Filtration and separation: The reaction solution after enzymolysis is first filtered with a filter press, and then separated with a separator, and the rotation speed is required to be 7600 rpm. Take the solid particles after separation.
[0038] 4. Drying: Bake the solid particles at 121°C for more than 2 hours to constant weight to obtain hemin. The purity of hemin is 90%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com