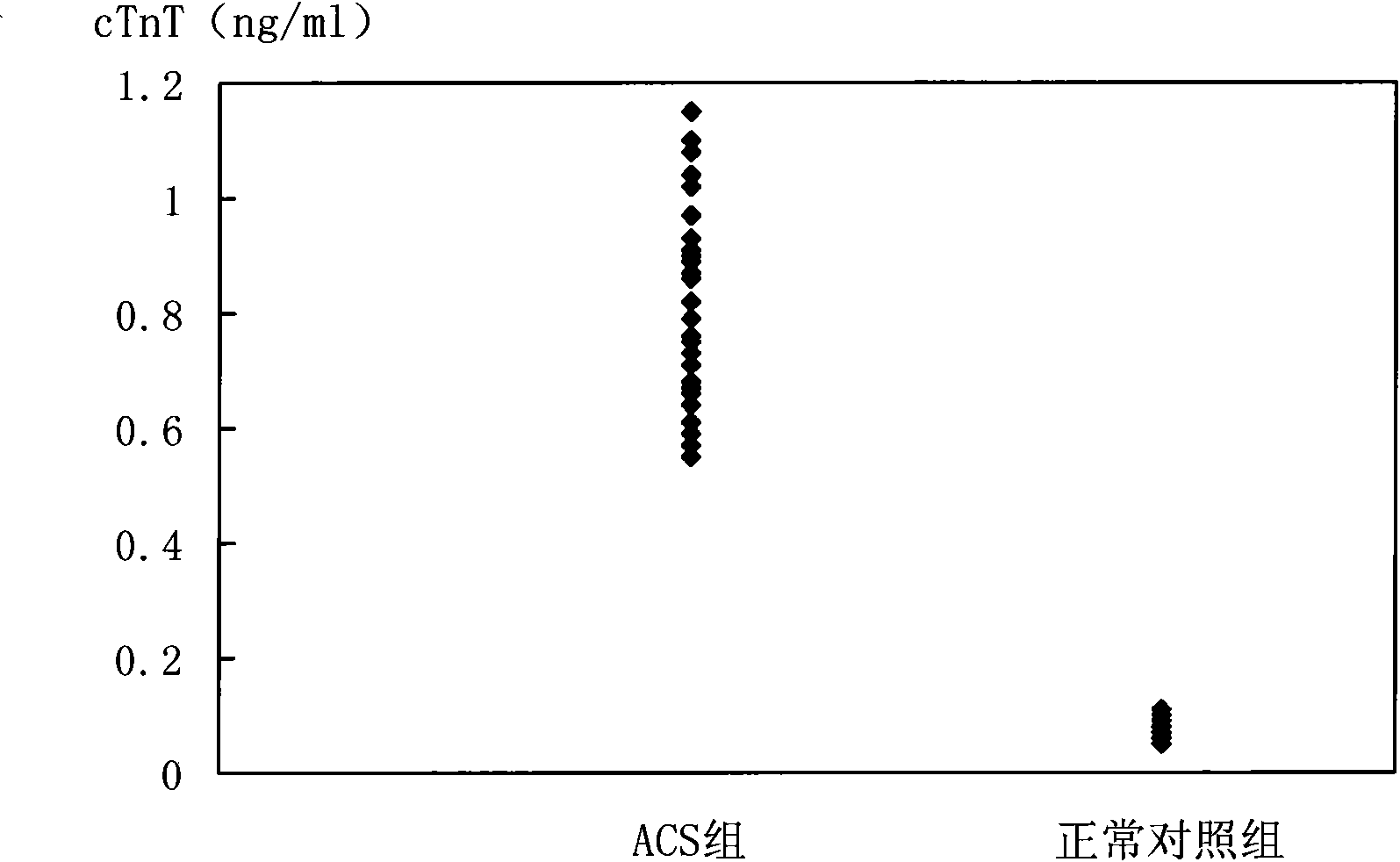
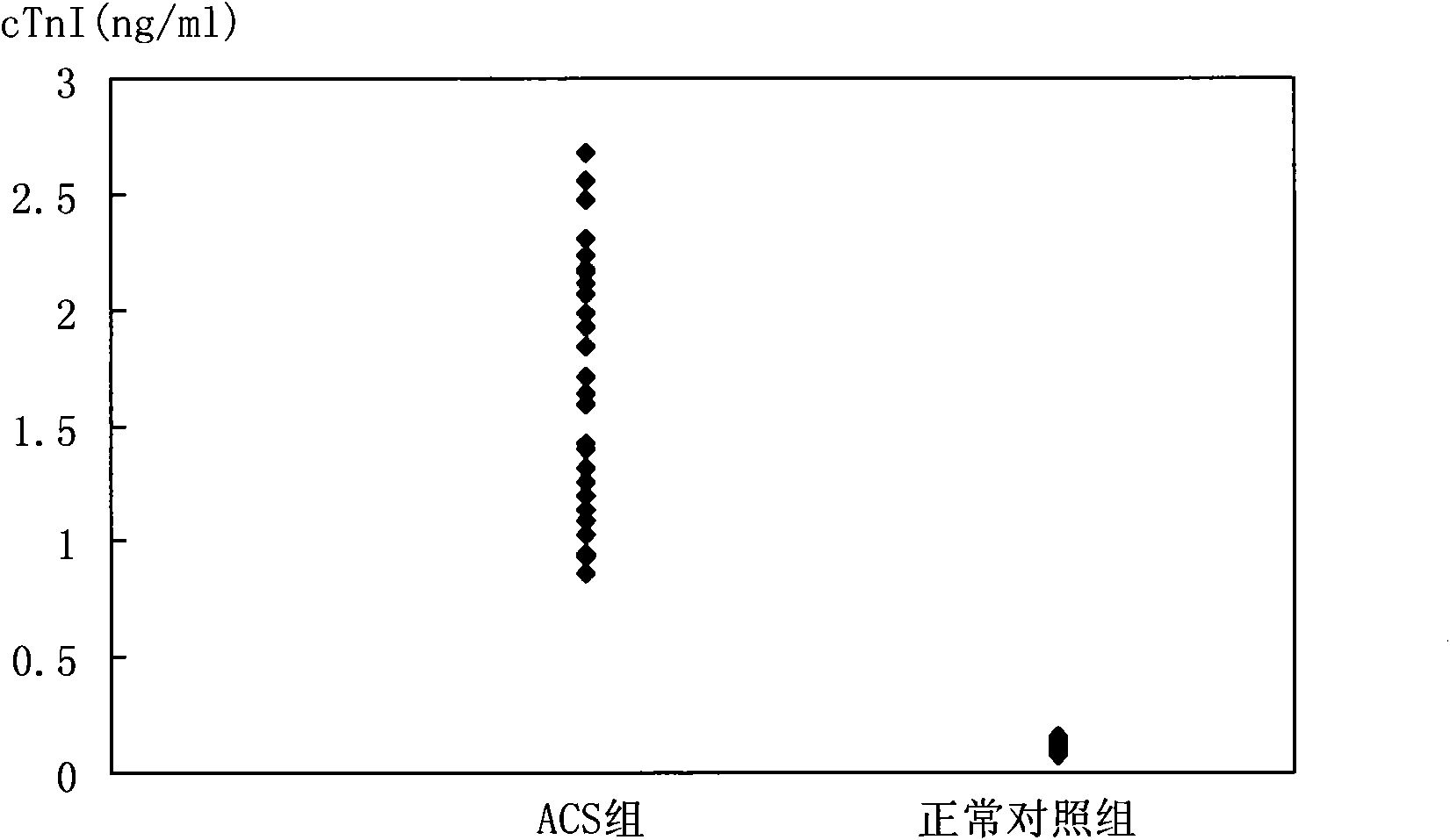
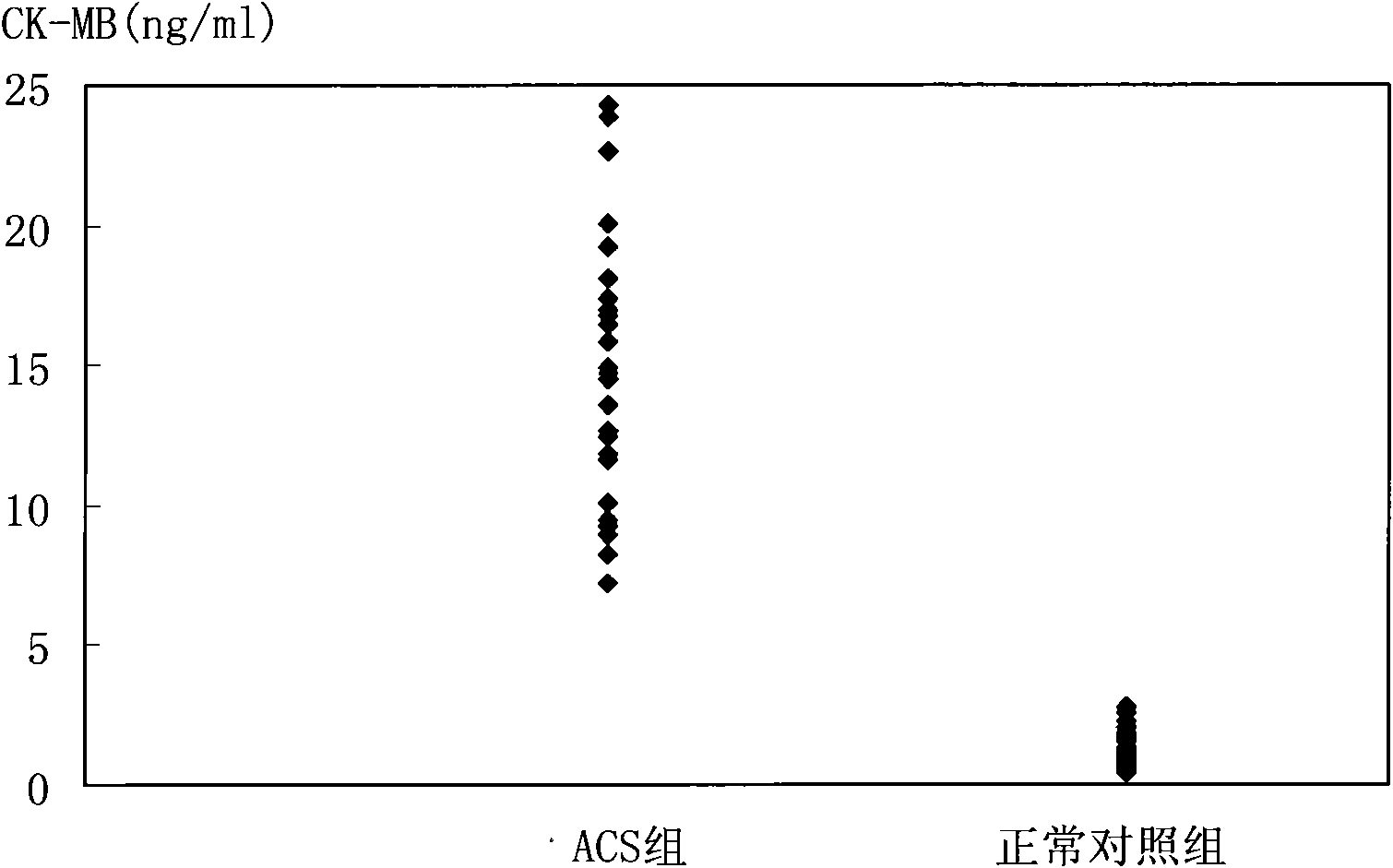
Biomarker detection method and diagnostic kit for acute coronary syndrome
A technology of biomarkers and diagnostic kits, which is applied in the field of liquid-phase chip combined with parallel detection methods and diagnostic kits, can solve the problems of poor repeatability, insufficient sensitivity, and cumbersome operation of solid-phase biochip technology, and achieve high Sensitivity, stability, and high-throughput effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Liquid-phase chip combined parallel detection method for three markers of acute coronary syndrome
[0045] The specific detection method includes the following steps:
[0046] 1. Activation of the required microspheres:
[0047] 1.1 Vortex the microsphere storage solution at full speed for at least 3 minutes to form a uniform microsphere suspension;
[0048] 1.2 Weigh 10 mg of EDC and S-NHS into two centrifuge tubes;
[0049] 1.3 Dissolve in deionized water to make the final concentration 50mg / ml;
[0050] 1.4 Take 1ml of the microsphere suspension and centrifuge at 10000g for 3min, carefully remove the supernatant;
[0051] 1.5 Add 80 μl of activation buffer to resuspend the microspheres;
[0052] 1.6 Add 10 μl of EDC solution (50 mg / ml) and 10 μl of S-NHS solution (50 mg / ml) respectively, mix well, and incubate at room temperature (15-25° C.) in the dark and shake for 20 minutes.
[0053] 2. Coupling of corresponding capture antibodies to activated mic...
Embodiment 2
[0099] Example 2: Liquid chip combined parallel detection method for six acute coronary syndrome markers The specific detection method, steps 1-3 are the same as in Example 1.
[0100] 4. Configuration of Antigen Standards
[0101] cTnT and cTnI are prepared according to the concentration of 31.25, 6.25, 1.25, 0.25, 0.05, 0.01, 0ng / ml, CK-MB and H-FABP are prepared according to the concentration of 312.5, 62.5, 12.5, 2.5, 0.5, 0.1, 0ng / ml For preparation, Myo and MPO were prepared at concentrations of 3125, 625, 125, 25, 5, 1, and 0 ng / ml, and the marker mixtures were labeled as STD6, STD5, STD4, STD3, STD2, STD1, and STD0, respectively.
[0102] 5. Preparation of microsphere mixture (mixture I) coupled with capture antibody
[0103] Take the microspheres coated with the capture antibodies of the 6 ACS markers, as follows: cTnT capture antibody microspheres 11, cTnI capture antibody microspheres 15, CK-MB capture antibody microspheres 21, H-FABP capture antibody microsphere...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com