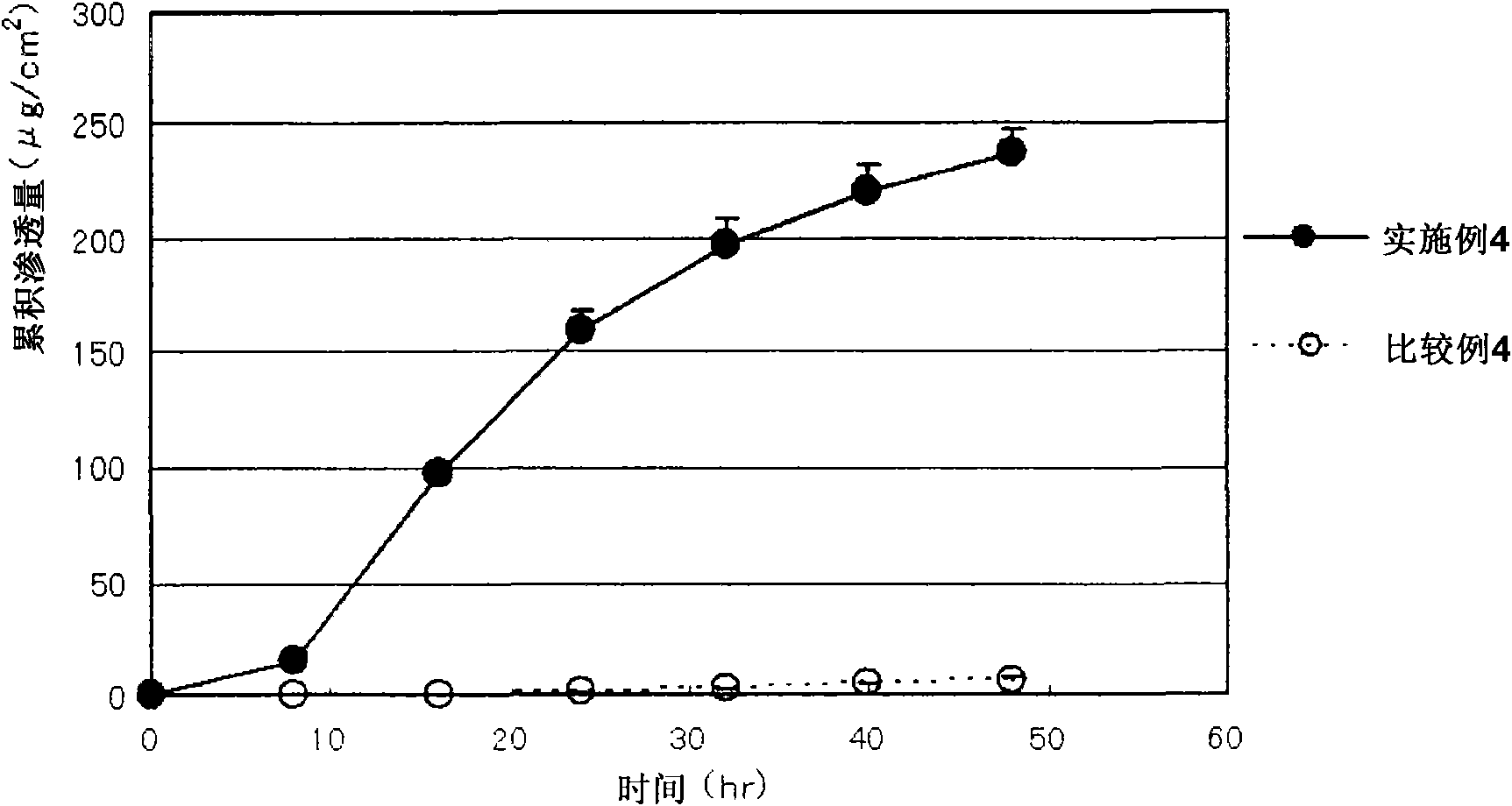
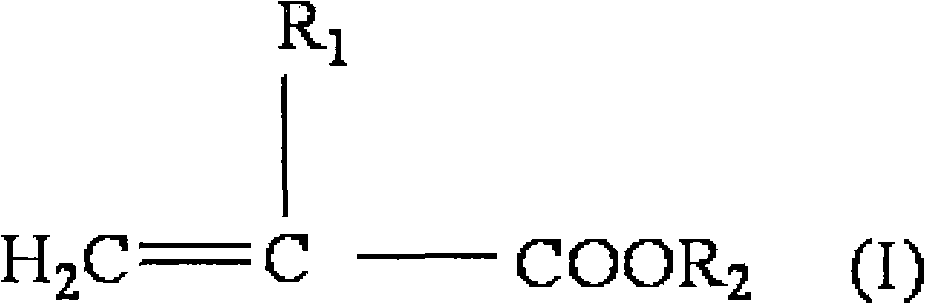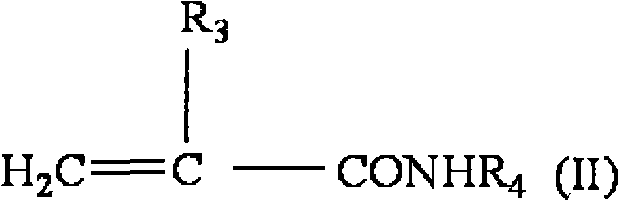Medical pressure-sensitive adhesive composition
A technology of composition and adhesive, which is applied in the direction of medical preparations of non-active ingredients, medical science, pharmaceutical formula, etc., can solve problems such as detachment, and achieve the effect of denaturation inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] [Example 1] Adhesive composition
[0071] First, 70 parts of 2-ethylhexyl acrylate (hereinafter referred to as "2-EHA") as monomer (a), 10 parts of N-hydroxyethylacrylamide as monomer (b) (hereinafter referred to as is "HEAA"), 20 parts of N-vinyl-2-pyrrolidone (hereinafter may be referred to as "N-VP") as a monomer (c), and 333.3 parts of ethyl acetate as a solvent are charged into a cooling tube, nitrogen guide tube, thermometer, dropping funnel, and stirrer, then the contents were stirred at room temperature for 1 h while nitrogen sparging (100 mL / min). After that, the contents in the reaction vessel were heated, and when the temperature of the contents reached 60°C, 0.2 parts of 2,2'-azobisisobutyronitrile (AIBN) was added as a polymerization initiator. The temperature of the contents was controlled so as to be kept at 60° C., and then polymerization was performed in a nitrogen stream for 6 hours. Next, the temperature was maintained at 76°C for 15 hours. Based o...
Embodiment 2
[0072] [Example 2] Adhesive composition
[0073] Using 85 parts of 2-ethylhexyl acrylate as monomer (a), 10 parts of N-hydroxyethylacrylamide as monomer (b) and 5 parts of N-vinyl-2 as monomer (c) - An adhesive composition (2-EHA / HEAA / N-VP=85 / 10 / 5) was prepared as an acrylic copolymer solution in the same manner as in Example 1 except that pyrrolidone was used as a monomer component. .
Embodiment 3
[0074] [Example 3] Adhesive composition
[0075] Using 50 parts of 2-ethylhexyl acrylate as monomer (a), 10 parts of N-hydroxyethylacrylamide as monomer (b) and 40 parts of N-vinyl-2 as monomer (c) - Pyrrolidone was used as a monomer component, and an adhesive composition as an acrylic copolymer solution (2-EHA / HEAA / N-VP=50 / 10 / 40 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com