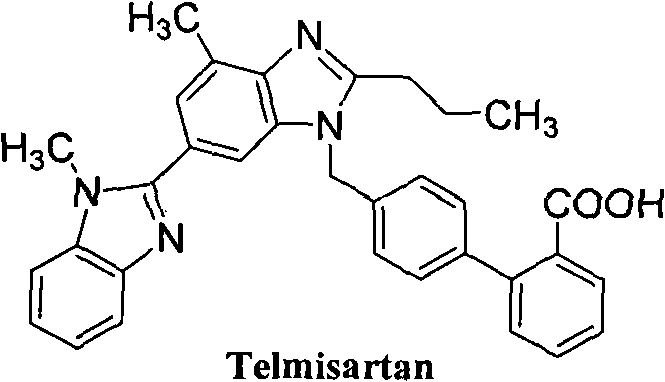
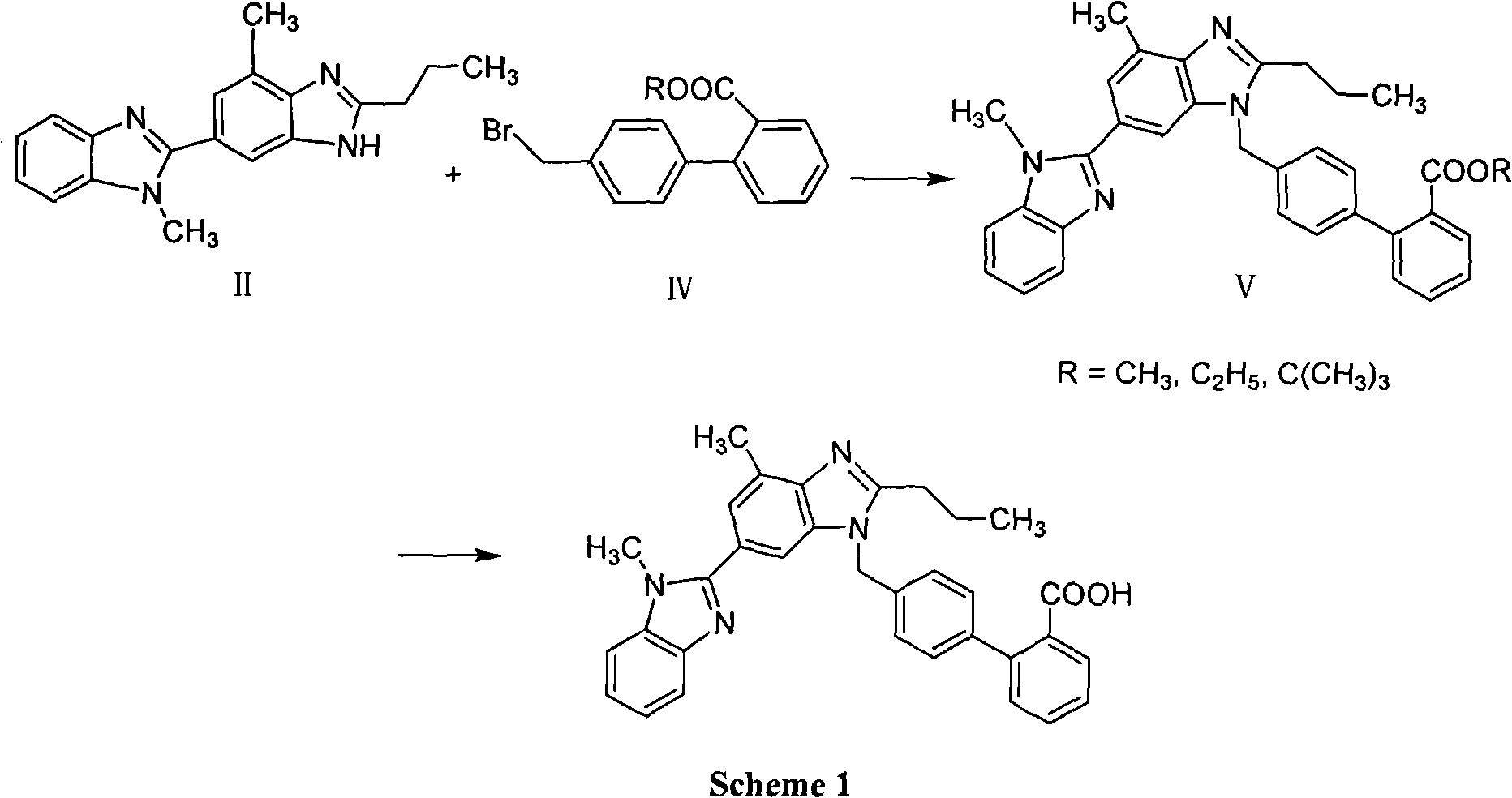
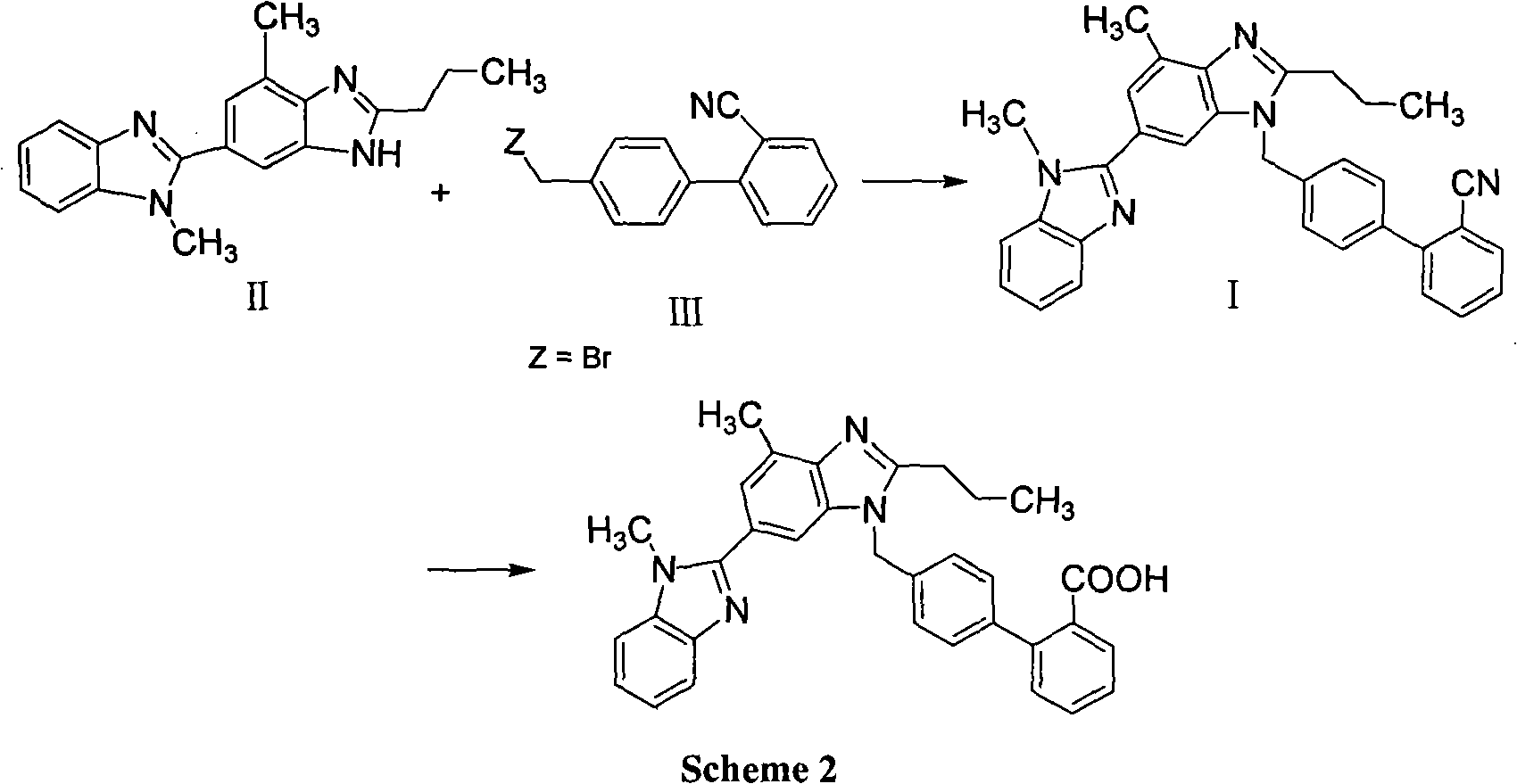
Method for preparing telmisartan intermediate
A technology for telmisartan and intermediates, which is applied in the field of intermediate synthesis, can solve problems such as the lack of practical significance in synthesis, and achieve the effects of good industrialization prospects, effective recovery and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] In a 250ml three-necked flask, add 8gNaOH, 13.5ml water, 1.0gTBAB and 11g2-n-propyl-4-methyl-6-(1'-methylbenzimidazol-2'-yl) benzimidazole, 40ml 1 , 2-dichloroethane. A mixture of 9g of 2-cyano-4'-bromomethylbiphenyl and 60ml of 1,2-dichloroethane was added dropwise while stirring under cooling at 0-10°C. After the dropwise addition was completed, the reaction was continued for 5 hours. After the reaction was completed, the layers were separated, and the organic layer was dried over anhydrous magnesium sulfate. The solvent was removed, the residue was washed with 100ml of tertiary methyl ether, washed with water, and dried in an oven to constant weight to obtain 2-cyano-4'-[2"-n-propyl-4"-methyl-6"-( 1'"-methylbenzimidazol-2'"-yl)benzimidazol-1"-ylmethyl]biphenyl, yield 92%, HPLC content 99.6%.
Embodiment 2
[0033] Add 20ml K to a 250ml three-neck flask 2 CO 3 Saturated solution, 0.9g TBAB and 11 grams of 2-n-propyl-4-methyl-6-(1'-methylbenzimidazol-2'-yl)benzimidazole, 40ml of 1,2-dichloroethane. A mixture of 9 g of 2-cyano-4'-bromomethylbiphenyl and 60 ml of 1,2-dichloroethane was added dropwise while stirring under cooling at -10°C-0°C. After the dropwise addition was completed, the reaction was continued for 6 hours. After the reaction was completed, the layers were separated, and the organic layer was dried over anhydrous magnesium sulfate. The solvent was removed, the residue was washed with 100ml of ether and then washed with water, and dried in an oven to constant weight to obtain 2-cyano-4'-[2"-n-propyl-4"-methyl-6"-(1' "-Methylbenzimidazol-2'"-yl)benzimidazol-1"-ylmethyl]biphenyl, yield 87%, HPLC content 99.2%.
Embodiment 3
[0035] In a 250ml three-necked flask, add 8gNaOH, 20ml water, 1.2gTBAHS and 11 grams of 2-n-propyl-4-methyl-6-(1'-methylbenzimidazol-2'-yl)benzimidazole, 40ml carbon tetrachloride. Add dropwise a mixture of 9g of 2-cyano-4'-bromomethylbiphenyl and 60ml of carbon tetrachloride while stirring under cooling at 0-10°C. After the dropwise addition was completed, the reaction was continued for 4 hours. After the reaction was completed, the layers were separated, and the organic layer was dried over anhydrous magnesium sulfate. The solvent was removed, the residue was washed with 100ml of petroleum ether, washed with water, and dried in an oven to constant weight to obtain 2-cyano-4'-[2"-n-propyl-4"-methyl-6"-(1 '"-methylbenzimidazol-2'"-yl)benzimidazol-1"-ylmethyl]biphenyl, yield 90.5%, HPLC content 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com