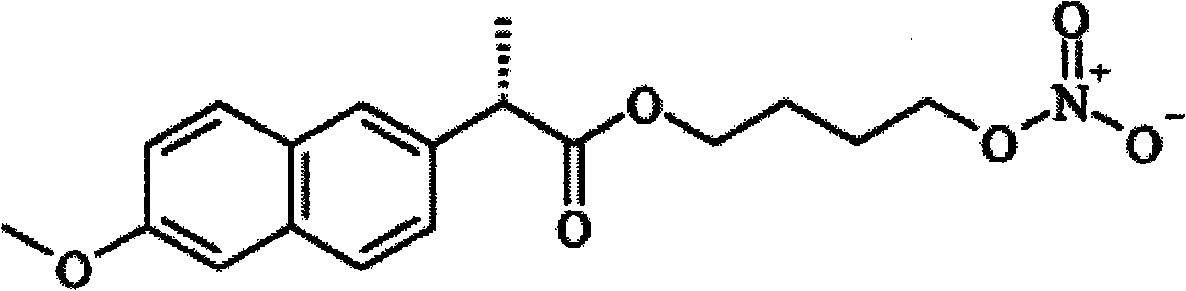
Method for the preparation of organic nitrates
A technology of nitric acid and esterification of hydroxyl groups, which is applied in the field of preparation of organic nitrates, and can solve the problems of not providing yields, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1: Preparation of 1,4-butanediol monoacetate with liquid catalyst
[0085] Add 1,4-butanediol (400g, 4.4mol), acetic acid (254.0g, 4.2mol) and CH to a 10L glass container equipped with a stirring blade at 20°C 2 Cl 2 (4.0kg). To this clear solution, 98% sulfuric acid (21.2 g, 0.22 mol) was added dropwise within 5 minutes, and then the resulting cloudy mixture was stirred at 20°C for 6 hours. Use 5% Na 2 CO 3 The reaction mixture was extracted with an aqueous solution (2x1300 mL) to remove unreacted acetic acid. The organic phase was then washed with water (2x500 mL). After removing the solvent under vacuum (35°C, 600 mbar), a liquid product (289.3 g) was obtained. GC analysis (FID): 1,4-butanediol monoacetate 91.8%-area.
Embodiment 2
[0086] Example 2: Preparation of 1,4-butanediol monoacetate with solid catalyst
[0087] Add 1,4-butanediol (240.1g, 2.7mol), acetic acid (152.0g, 2.5mol), CH to a 10L glass container equipped with a stirring blade and a reflux condenser at 20°C 2 Cl 2 (1.2kg) and amberlyst 36 (12.0g). The resulting clear mixture was heated to reflux. After 24 hours, cool the mixture, filter off the solid catalyst, and use 5% Na 2 CO 3 The organic phase was washed with aqueous solution (2*400 mL) and water (2*170 mL). The solvent was removed in vacuo to obtain 179.4 g of clear liquid. According to GC analysis (FID), 1,4-butanediol monoacetate was obtained with a yield of 92.0%.
Embodiment 3
[0088] Example 3: Preparation of 4-hydroxybutyl 4-nitrobenzoate
[0089] Under nitrogen atmosphere, add 1,4-butane-diol (90.0g, 1.0mol, 3.7eq.), 4-(dimethylamino)-pyridine (DMAP, 0.3g), acetone (300mL) and triethylamine in sequence (23.4g) and cooled to -5°C. The p-nitrobenzoyl chloride (37.1 g, 0.27 mol, 1 eq.) in acetone (200 mL) was added dropwise while maintaining the internal temperature 2 Cl 2 (500 mL) was added to the residue and the resulting mixture was washed with brine (3x300 mL). Take Na 2 SO 4 The organic residue was dried, filtered and the solvent was removed under reduced pressure to dryness. The resulting product 4-hydroxybutyl 4-nitrobenzoate was a yellow solid (31.5 g, 66%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com