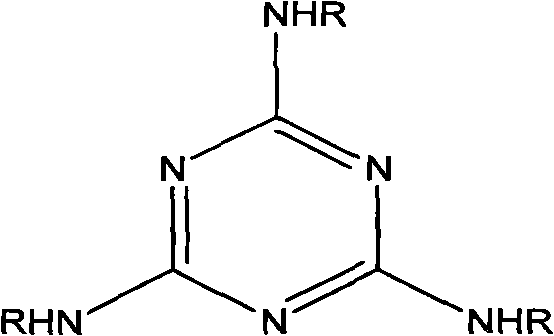
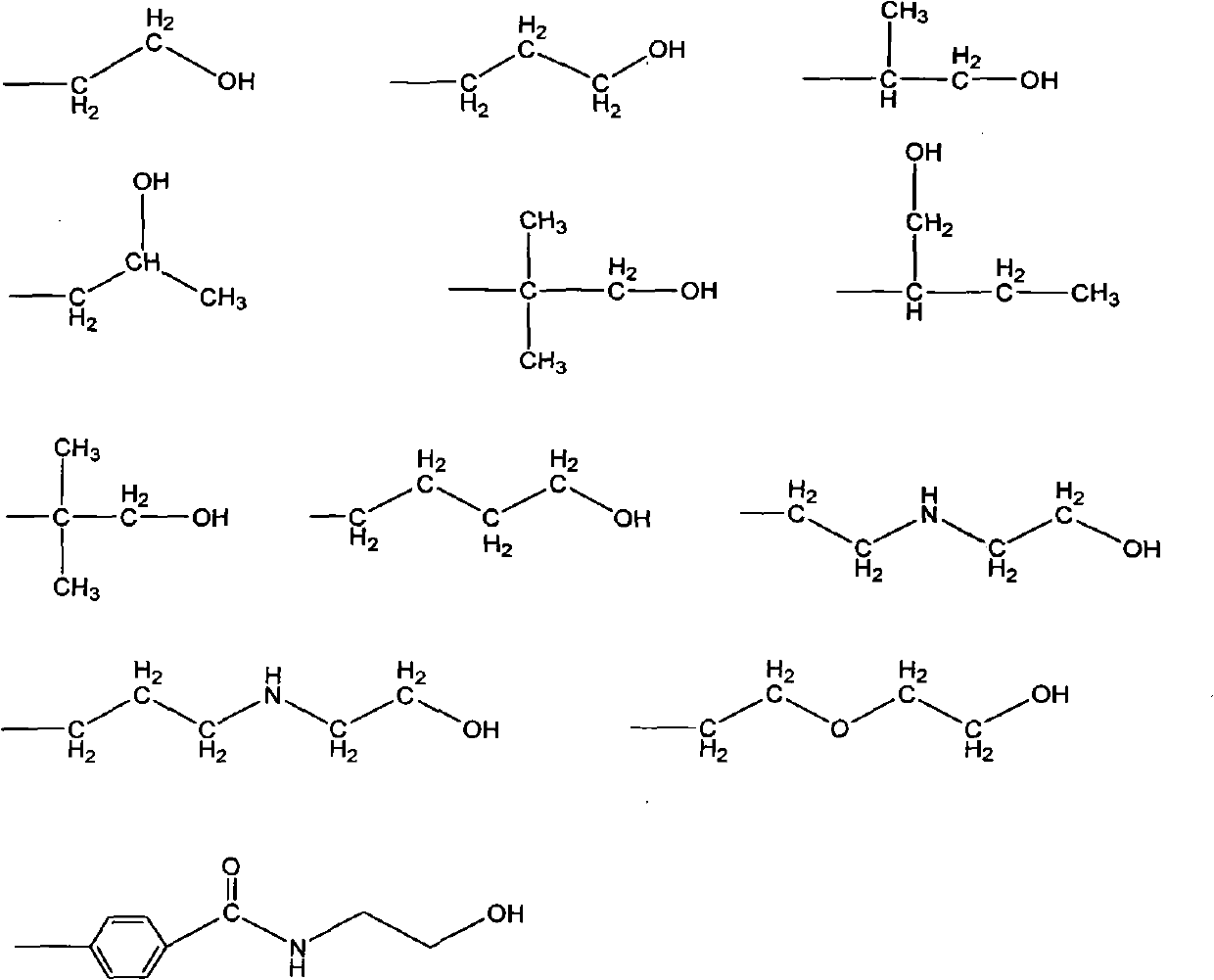
Synthetic method of triazine derivative
The technology of a triazine derivative and a production method, which is applied in the chemical industry, can solve the problems of high reaction temperature, low yield, long reaction time and the like, and achieve the effects of simple synthesis method, wide application range and easy production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 1 mole of 184 grams of cyanuric chloride, 10 moles of 1260 grams of melamine and 30 moles of 1832 grams of 2-aminoethanol in the reaction kettle, and simultaneously introduce a weak nitrogen flow, heat up to 100-120 ° C, and react for 30 minutes under stirring. Then the temperature was raised to 150-160° C. to react for 3 hours. The whole reaction process is protected by a weak nitrogen flow. After the synthesis is completed, add 3 moles of 50% sodium hydroxide solution and stir for about 15 minutes, then turn on vacuum distillation to concentrate, keep the temperature at 150-160, and then cool down until the reaction The temperature of the product is lower than 100, and the product is in the form of light yellow resin.
[0025] As detected by HPLC, the mole percentages of mono-substitution, di-substitution and tri-substitution in the mixture of triazine derivatives are 15mol%: 40mol%: 45mol%.
Embodiment 2
[0027] Add 3 moles of 552 grams of cyanuric chloride, 10 moles of 1260 grams of melamine and 30 moles of 2670 grams of 4-aminobutanol in the synthesis reactor. The reaction process and reaction time are the same as in Example 1.
[0028] As detected by HPLC, the molar percentages of monosubstituted, disubstituted and trisubstituted triazine derivatives in the mixture of triazine derivatives are: 5mol%: 40mol%: 55mol%.
Embodiment 3
[0030] Add 3 moles of 552 grams of cyanuric chloride, 10 moles of 1260 grams of melamine and 50 moles of 5257 grams of 2-(2-aminoethoxy) ethanol in the synthesis reactor, the reaction process is the same as in Example 1, and the synthesis reaction time is 5 Hours, neutralize the reaction with 4.5 molar sodium carbonate solution.
[0031] As detected by HPLC, the molar percentages of disubstituted and trisubstituted triazine derivatives in the mixture of triazine derivatives are: 30mol%: 70mol%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com