A kind of triazine compound and its application in controlling chicken coccidiosis
A triazine compound and the technology of the compound are applied in the triazine compound and its application field in the control of chicken coccidiosis, which can solve the problems of increasing the difficulty and inconvenience of preparation preparation, high concentration of use, and easy drug resistance, etc., and achieve Good preventive and curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
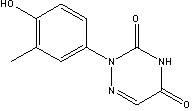
[0033] 2-{3-methyl-4-(4'-nitrophenoxy)phenyl}-1,2,4-triazine-3,5(2H,4H)-dione (compound of formula (I) ) preparation
[0034] Add compound Ⅱ (15 grams, 0.068mol), anhydrous sodium carbonate (7.9 grams, 0.075mol), p-chloronitrobenzene (11.8 grams, 0.075mol) and DMF (150ml) into a four-necked flask and heat to 120°C After reacting for 10 hours, after cooling down to room temperature, the reaction solution was poured into 1000ml of water, adjusted to pH=3 with 10% dilute hydrochloric acid, and a pale yellow solid was precipitated, filtered, washed with water, and dried to obtain the compound of formula (I) (16.2 g, 69.5%)
[0035] mp: 169 – 171.5°C. ESI-MS (m / z): 339.2(M-H) - , 1HNMR (CDCl 3): 2.25 (s, 3H), 7.00 (d, 2H), 7.09 (d, 1H), 7.44(d,1H), 7.50(s,1H), 7.60(s,1H), 8.22(d,2H) , 9.69 (s, 1H).
Embodiment 2
[0037] 2-{3-methyl-4-(4'-nitrophenoxy)phenyl}-1,2,4-triazine-3,5(2H,4H)-dione (compound of formula (I) ) preparation
[0038] Compound II (15 g, 0.068 mol), anhydrous potassium carbonate (10.4 g, 0.075 mol), p-chloronitrobenzene (11.8 g, 0.075 mol) and DMSO (100 ml), were added to a four-necked flask and heated to 130°C After reacting for 8 hours, after cooling down to room temperature, the reaction solution was poured into 1000ml of water, adjusted to pH=3 with 10% dilute sulfuric acid, and a pale yellow solid was precipitated, filtered, washed with water, and dried to obtain the compound of formula (I) (15 g, 64.3%).
Embodiment 3
[0040] Preparation of 2-(3-methyl-4-hydroxyphenyl)-1,2,4-triazine-3,5(2H,4H)-diketonol sodium salt
[0041] Compound Ⅱ (150 g, 0.68 mol), 500 g toluene, heated to dissolve, 30% sodium methoxide solution was added dropwise, a yellow powder was precipitated, filtered, washed with methanol, and dried to obtain 2-(3-methyl-4-hydroxybenzene base)-1,2,4-triazine-3,5(2H,4H)-diketone sodium salt (147.5 g, 90%).
[0042] ESI-MS (m / z): 218.1(M-Na) - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com